
| ʵ��ǰ | ʵ��� | |
| ����ͭ+������ | 20g | 16.8g |
| �Ȼ���+����� | 100.8g | 104.4g |
| ||
| 80 |
| 18 |
| x |
| 3.6g |
| 16g |
| 20g |


 �Ķ��쳵ϵ�д�
�Ķ��쳵ϵ�д�
| �꼶 | ���пγ� | �꼶 | ���пγ� |
| ��һ | ��һ��ѿγ��Ƽ��� | ��һ | ��һ��ѿγ��Ƽ��� |
| �߶� | �߶���ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
| ���� | ������ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
��Ŀ�����л�ѧ ��Դ�� ���ͣ�

| ʵ��ǰ | ʵ��� | |
| ������ͭ+�����ܣ�������/g | 75.6 | 69.2 |
| ��������+U�ܣ�������/g | 110.8 | 118.0 |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
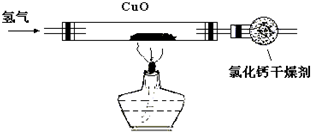
 ijѧУ̽��ѧϰС�飬���ô�����������ԭ����ͭ�����ʲ���������Ӧ�����ⶨ����ͭ�Ĵ��ȣ�װ����ͼ��ʾ�����õ��������ݣ�
ijѧУ̽��ѧϰС�飬���ô�����������ԭ����ͭ�����ʲ���������Ӧ�����ⶨ����ͭ�Ĵ��ȣ�װ����ͼ��ʾ�����õ��������ݣ�| ʵ��ǰ | ʵ��� | |
| A������ͭ��Ʒ | 40.0�� | 34.0�� |
| B���Ȼ���+U�� | 100.8�� | 108.0�� |
| ||
| ||
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ��������ѧ(��ѧ��ѧ������ѵ���������) ���ͣ�013
���������ȵ�����ͭ��Ӧ����Ҫ������������
[����]
|
A�������� |
B����ȼ�� |
|
C����ԭ�� |
D���ȶ��� |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�013
���������ȵ�����ͭ��Ӧ����Ҫ������������
[����]
|
A �������� |
B ����ȼ�� |
|
C ����ԭ�� |
D ���ȶ��� |
�鿴�𰸺ͽ���>>
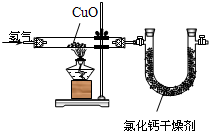
��Ŀ�����л�ѧ ��Դ��2001���ʮһ�조��ԭ����ȫ������ѧ����ѧ���ʺ�ʵ���������������Ծ��������棩 ���ͣ������
| ʵ��ǰ | ʵ��� | |
| ������ͭ+�����ܣ�������/g | 75.6 | 69.2 |
| ��������+U�ܣ�������/g | 110.8 | 118.0 |
�鿴�𰸺ͽ���>>
����ʡ������Υ���Ͳ�����Ϣ�ٱ�ƽ̨ | �����к���Ϣ�ٱ�ר�� | ����թƭ�ٱ�ר�� | ����ʷ���������к���Ϣ�ٱ�ר�� | ������Ȩ�ٱ�ר��
Υ���Ͳ�����Ϣ�ٱ��绰��027-86699610 �ٱ����䣺58377363@163.com