
| “ĪŹż | µŚ1“Ī | µŚ2“Ī | µŚ3“Ī |
¼ÓČėŃĪĖįµÄÖŹĮæ/g | 20 | 20 | 20 |
| ÉÕ±ÖŠŹ£ÓąĪļµÄÖŹĮæ/g | 38.24 | a | 74.72 |

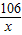 =
=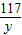 =
=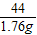
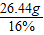 =165.25g£»
=165.25g£»


| Äź¼¶ | øßÖŠæĪ³Ģ | Äź¼¶ | ³õÖŠæĪ³Ģ |
| øßŅ» | øßŅ»Ćā·ŃæĪ³ĢĶĘ¼ö£” | ³õŅ» | ³õŅ»Ćā·ŃæĪ³ĢĶĘ¼ö£” |
| ø߶ž | ø߶žĆā·ŃæĪ³ĢĶĘ¼ö£” | ³õ¶ž | ³õ¶žĆā·ŃæĪ³ĢĶĘ¼ö£” |
| øßČż | øßČżĆā·ŃæĪ³ĢĶĘ¼ö£” | ³õČż | ³õČżĆā·ŃæĪ³ĢĶĘ¼ö£” |
æĘÄæ£ŗ³õÖŠ»Æѧ Ą“Ō“£ŗ ĢāŠĶ£ŗ
 ĻÖÓŠŅ»Ęæ“æ¼īѳʷĘäÖŠŗ¬ÓŠÉŁĮæNaClŌÓÖŹ£¬æĪĶā»ÆѧŠ”×éĶ¬Ń§½«100gŃĪĖį·Ö5“Ī¼ÓČėµ½20g“æ¼īѳʷ֊£¬µĆµ½ČēĶ¼²æ·ÖŹż¾ŻŗĶĶ¼Ļó£Ø¼ŁÉč²śÉśµÄĘųĢåČ«²æŅŻ³ö£©£®
ĻÖÓŠŅ»Ęæ“æ¼īѳʷĘäÖŠŗ¬ÓŠÉŁĮæNaClŌÓÖŹ£¬æĪĶā»ÆѧŠ”×éĶ¬Ń§½«100gŃĪĖį·Ö5“Ī¼ÓČėµ½20g“æ¼īѳʷ֊£¬µĆµ½ČēĶ¼²æ·ÖŹż¾ŻŗĶĶ¼Ļó£Ø¼ŁÉč²śÉśµÄĘųĢåČ«²æŅŻ³ö£©£®| “ĪŹż | µŚ1“Ī | µŚ2“Ī | µŚ3“Ī |
¼ÓČėŃĪĖįµÄÖŹĮæ/g |
20 | 20 | 20 |
| ÉÕ±ÖŠŹ£ÓąĪļµÄÖŹĮæ/g | 38.24 | a | 74.72 |
²éæ““š°øŗĶ½āĪö>>
æĘÄæ£ŗ³õÖŠ»Æѧ Ą“Ō“£ŗ2010½ģ±±¾©ŹŠĘ½¹ČĒųÖŠæ¼Ņ»Ä£»ÆѧŹŌ¾ķ£Ø“ų½āĪö£© ĢāŠĶ£ŗ¼ĘĖćĢā
£Ø3·Ö£©ĻÖÓŠŅ»Ęæ“æ¼īѳʷĘäÖŠŗ¬ÓŠÉŁĮæNaClŌÓÖŹ£¬æĪĶā»ÆѧŠ”×éĶ¬Ń§½«100gŃĪĖį·Ö5“Ī¼ÓČėµ½20g“æ¼īѳʷ֊£¬µĆµ½ČēĻĀ²æ·ÖŹż¾ŻŗĶĶ¼Ļó(¼ŁÉč²śÉśµÄĘųĢåČ«²æŅŻ³ö)”£
| “ĪŹż | µŚ1“Ī | µŚ2“Ī | µŚ3“Ī |
| ¼ÓČėŃĪĖįµÄÖŹĮæ/g | 20 | 20 | 20 |
| ÉÕ±ÖŠŹ£ÓąĪļµÄÖŹĮæ/g | 38.24 | a | 74.72 |
²éæ““š°øŗĶ½āĪö>>
æĘÄæ£ŗ³õÖŠ»Æѧ Ą“Ō“£ŗ2010½ģ±±¾©ŹŠĘ½¹ČĒųÖŠæ¼Ņ»Ä£»ÆѧŹŌ¾ķ£Ø½āĪö°ę£© ĢāŠĶ£ŗ¼ĘĖćĢā
£Ø3·Ö£©ĻÖÓŠŅ»Ęæ“æ¼īѳʷĘäÖŠŗ¬ÓŠÉŁĮæNaClŌÓÖŹ£¬æĪĶā»ÆѧŠ”×éĶ¬Ń§½«100gŃĪĖį·Ö5“Ī¼ÓČėµ½20g“æ¼īѳʷ֊£¬µĆµ½ČēĻĀ²æ·ÖŹż¾ŻŗĶĶ¼Ļó(¼ŁÉč²śÉśµÄĘųĢåČ«²æŅŻ³ö)”£

|
“ĪŹż |
µŚ1“Ī |
µŚ2“Ī |
µŚ3“Ī |
|
¼ÓČėŃĪĖįµÄÖŹĮæ/g |
20 |
20 |
20 |
|
ÉÕ±ÖŠŹ£ÓąĪļµÄÖŹĮæ/g |
38.24 |
a |
74.72 |
Ēė¼ĘĖć£ŗ
£Ø1£©µŚ2“Ī¼ÓČėŃĪĖįŗó£¬aĪŖ___ ____g”£
£Ø2£©16%µÄNaClČÜŅŗæÉÓĆÓŚÉśĪļŠ”×é½ųŠŠŃ”ÖÖ”£Óū½«µŚ5“ĪŹµŃéŗóµÄČÜŅŗÅä³É16%µÄNaClČÜŅŗ£¬æÉĻČĻņ“ĖČÜŅŗÖŠ¼ÓČėŹŹĮæµÄĢ¼ĖįÄĘ·ŪÄ©£¬ÖĮĪŽĘųÅŻ²śÉśĪŖÖ¹£¬ÕāŹ±»¹ŠčŅŖĻņČÜŅŗÖŠ¼ÓČėĖ®¶ąÉŁæĖ£æ£Ø¼ŁÉ菵Ńé¹ż³ĢÖŠČÜŅŗĖšŹ§ŗöĀŌ²»¼Ę£©
²éæ““š°øŗĶ½āĪö>>
æĘÄæ£ŗ³õÖŠ»Æѧ Ą“Ō“£ŗ ĢāŠĶ£ŗ½ā“šĢā
 ĻÖÓŠŅ»Ęæ“æ¼īѳʷĘäÖŠŗ¬ÓŠÉŁĮæNaClŌÓÖŹ£¬æĪĶā»ÆѧŠ”×éĶ¬Ń§½«100gŃĪĖį·Ö5“Ī¼ÓČėµ½20g“æ¼īѳʷ֊£¬µĆµ½ČēĶ¼²æ·ÖŹż¾ŻŗĶĶ¼Ļó£Ø¼ŁÉč²śÉśµÄĘųĢåČ«²æŅŻ³ö£©£®
ĻÖÓŠŅ»Ęæ“æ¼īѳʷĘäÖŠŗ¬ÓŠÉŁĮæNaClŌÓÖŹ£¬æĪĶā»ÆѧŠ”×éĶ¬Ń§½«100gŃĪĖį·Ö5“Ī¼ÓČėµ½20g“æ¼īѳʷ֊£¬µĆµ½ČēĶ¼²æ·ÖŹż¾ŻŗĶĶ¼Ļó£Ø¼ŁÉč²śÉśµÄĘųĢåČ«²æŅŻ³ö£©£®| “ĪŹż | µŚ1“Ī | µŚ2“Ī | µŚ3“Ī |
¼ÓČėŃĪĖįµÄÖŹĮæ/g | 20 | 20 | 20 |
| ÉÕ±ÖŠŹ£ÓąĪļµÄÖŹĮæ/g | 38.24 | a | 74.72 |
²éæ““š°øŗĶ½āĪö>>
°Ł¶ČÖĀŠÅ - Į·Ļ°²įĮŠ±ķ - ŹŌĢāĮŠ±ķ
ŗž±±Ź”»„ĮŖĶųĪ„·ØŗĶ²»Į¼ŠÅĻ¢¾Ł±ØĘ½ĢØ | ĶųÉĻÓŠŗ¦ŠÅĻ¢¾Ł±Ø×ØĒų | µēŠÅÕ©Ę¾Ł±Ø×ØĒų | É꥜Ź·ŠéĪŽÖ÷ŅåÓŠŗ¦ŠÅĻ¢¾Ł±Ø×ØĒų | ÉęĘóĒÖČؾŁ±Ø×ØĒų
Ī„·ØŗĶ²»Į¼ŠÅĻ¢¾Ł±Øµē»°£ŗ027-86699610 ¾Ł±ØÓŹĻä£ŗ58377363@163.com