

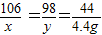
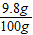 ×100%=9.8%
×100%=9.8%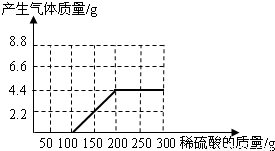


 ��ѧ��������������Ͼ���ѧ������ϵ�д�
��ѧ��������������Ͼ���ѧ������ϵ�д� �ϴ�̸�������������νӽ̳��Ͼ���ѧ������ϵ�д�
�ϴ�̸�������������νӽ̳��Ͼ���ѧ������ϵ�д�
| �꼶 | ���пγ� | �꼶 | ���пγ� |
| ��һ | ��һ��ѿγ��Ƽ��� | ��һ | ��һ��ѿγ��Ƽ��� |
| �߶� | �߶���ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
| ���� | ������ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
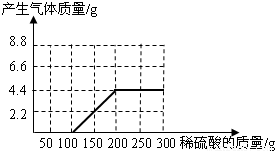
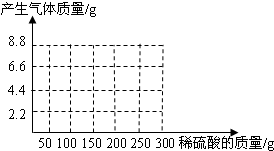 ��NaOH��Na2CO3�����l8.6g��Ϊ�ⶨ����NaOH������������������ˮ��������ϡ������100gʱ����ʼ�������壻�ټ���ϡ������100gʱ�����ٲ������壬�������干4.4g
��NaOH��Na2CO3�����l8.6g��Ϊ�ⶨ����NaOH������������������ˮ��������ϡ������100gʱ����ʼ�������壻�ټ���ϡ������100gʱ�����ٲ������壬�������干4.4g�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�

| ʵ�鲽�� | ʵ������ | ʵ����� |
| ȡ�����ķ�Һ���ˣ�����Һ�м�������� CaCl2 CaCl2 ��Һ |
�� ��Һ�г��ְ�ɫ���� ��Һ�г��ְ�ɫ���� �� ��Һ�����ɫ ��Һ�����ɫ |
����ݳ��� |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ��2011����б�ҵ��ѧ���ԣ����ݹ���������ѧ���� ���ͣ�������
��NaOH��Na2CO3�����l8.6g��Ϊ�ⶨ����NaOH������������������ˮ��������ϡ������100gʱ����ʼ�������壻�ټ���ϡ������100gʱ�����ٲ������壬�������干4.4g(��ʾ��Na2CO3+ H2SO4 = Na2SO4+ CO2��+H2O)
(1)��ͼ28�л����������������������ϡ���������Ĺ�ϵ���ߡ�
(2)��������NaOH�������Ƕ���?
(3)����ϡ������������������Ƕ��٣�
�鿴�𰸺ͽ���>>
����ʡ������Υ���Ͳ�����Ϣ�ٱ�ƽ̨ | �����к���Ϣ�ٱ�ר�� | ����թƭ�ٱ�ר�� | ����ʷ���������к���Ϣ�ٱ�ר�� | ������Ȩ�ٱ�ר��
Υ���Ͳ�����Ϣ�ٱ��绰��027-86699610 �ٱ����䣺58377363@163.com