
���� ��1��������Һϡ��ǰ�����ʵ��������������
��2�����ݷ�Ӧ�Ļ�ѧ����ʽ����ǡ����ȫ��Ӧ�����������������������̼��Ƶ�����������������
��� �⣺��1��������Һϡ��ǰ�����ʵ�������������24%����������Ϊx�ɵã�
24%��x��1.1g/cm3=240mL��1.0g/cm3��7.3%
x=66.4mL��
��2����μӷ�Ӧ��̼��Ƶ�����Ϊx��
CaCO3+2HCl�TCaCl2+H2O+CO2��
100 73
x 100g��7.3%
$\frac{100}{x}$=$\frac{73}{100g��7.3%}$
x=10g
��������̼��Ƶ���������$\frac{10g}{12g}$��100%��83.3%
�ʴ�Ϊ����1��66.4mL��
��2��83.3%��
���� ���ݻ�ѧ����ʽ�ļ��㣬��ʹ�����ʵ���������Ϊ������������������ʵ����ʵ���������ת��Ϊ��������������ܴ��뻯ѧ����ʽ�ļ��㣮


 ��ʱѵ���������������ϵ�д�
��ʱѵ���������������ϵ�д�
| �꼶 | ���пγ� | �꼶 | ���пγ� |
| ��һ | ��һ��ѿγ��Ƽ��� | ��һ | ��һ��ѿγ��Ƽ��� |
| �߶� | �߶���ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
| ���� | ������ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
��Ŀ�����л�ѧ ��Դ�� ���ͣ������
| �� �� | �� �� | �� �� �� �� | �� �� �� Ӧ |
| 101�桫102�� | 150�桫160�� ���� | 100.1��ֽ��ˮ��175��ֽ��CO2��CO��H2O | �� Ca��OH��2��Ӧ������ɫ������CaC2O4�� |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�ѡ����
| A�� | ���ó����Ǿ���ˮ�̶���ߵķ��� | B�� | �÷���ˮ���Լ���Ӳˮ����ˮ | ||
| C�� | ����ʹ��ũҩ�����ˮ����Ⱦ | D�� | ��ˮ���������Ȼ��ƺ��������� |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ������
 ��1���û�ѧ������գ�
��1���û�ѧ������գ��鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ������
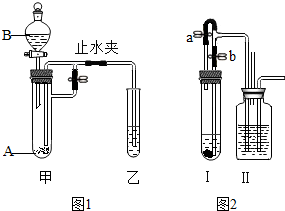 ij��ѧС��ͬѧ����ͼʾװ�ý���ʵ�飨ͼ�й̶�װ��ʡ�ԣ�������װ�ü��Թ���ʢ�й����ĩA����Һ©����ʢ��������ҺB��
ij��ѧС��ͬѧ����ͼʾװ�ý���ʵ�飨ͼ�й̶�װ��ʡ�ԣ�������װ�ü��Թ���ʢ�й����ĩA����Һ©����ʢ��������ҺB���鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�ѡ����
| A�� | NaOH ��� | B�� | Ca��OH��2 ��ʯ�� | C�� | NaHCO3 С�մ� | D�� | CaCO3ʯ��ʯ |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ������
 ������ʳƷ�ල�����ֹ������������һ����ʳƷ������ݣ��ڳ���18����������Ʒ�У���8���β�Ʒ�ӳ��꣮��������ɫ�н�������Ľ��������ڳ�ʪ�����л���������ʧȥ����������ʱ�����γ�������㣬�����Ӻ�ǿ�����ӵ��ܽ�ȶ���С�����������ᣬ�������ڼ�����Ķ����ϲ��ϻش�һ�����⣺
������ʳƷ�ල�����ֹ������������һ����ʳƷ������ݣ��ڳ���18����������Ʒ�У���8���β�Ʒ�ӳ��꣮��������ɫ�н�������Ľ��������ڳ�ʪ�����л���������ʧȥ����������ʱ�����γ�������㣬�����Ӻ�ǿ�����ӵ��ܽ�ȶ���С�����������ᣬ�������ڼ�����Ķ����ϲ��ϻش�һ�����⣺ ���û�ѧʽ����ʽ�С�
���û�ѧʽ����ʽ�С� ���ڶ�Ӧ�Ļ�ѧʽΪH2O��
���ڶ�Ӧ�Ļ�ѧʽΪH2O���鿴�𰸺ͽ���>>
����ʡ������Υ���Ͳ�����Ϣ�ٱ�ƽ̨ | �����к���Ϣ�ٱ�ר�� | ����թƭ�ٱ�ר�� | ����ʷ���������к���Ϣ�ٱ�ר�� | ������Ȩ�ٱ�ר��
Υ���Ͳ�����Ϣ�ٱ��绰��027-86699610 �ٱ����䣺58377363@163.com