



| Äź¼¶ | øßÖŠæĪ³Ģ | Äź¼¶ | ³õÖŠæĪ³Ģ |
| øßŅ» | øßŅ»Ćā·ŃæĪ³ĢĶĘ¼ö£” | ³õŅ» | ³õŅ»Ćā·ŃæĪ³ĢĶĘ¼ö£” |
| ø߶ž | ø߶žĆā·ŃæĪ³ĢĶĘ¼ö£” | ³õ¶ž | ³õ¶žĆā·ŃæĪ³ĢĶĘ¼ö£” |
| øßČż | øßČżĆā·ŃæĪ³ĢĶĘ¼ö£” | ³õČż | ³õČżĆā·ŃæĪ³ĢĶĘ¼ö£” |
æĘÄæ£ŗ³õÖŠ»Æѧ Ą“Ō“£ŗ ĢāŠĶ£ŗ
²éæ““š°øŗĶ½āĪö>>
æĘÄæ£ŗ³õÖŠ»Æѧ Ą“Ō“£ŗ ĢāŠĶ£ŗ
²éæ““š°øŗĶ½āĪö>>
æĘÄæ£ŗ³õÖŠ»Æѧ Ą“Ō“£ŗ ĢāŠĶ£ŗ
| ŹµŃé²½Öč¼°ĻÖĻó | ½įĀŪ |
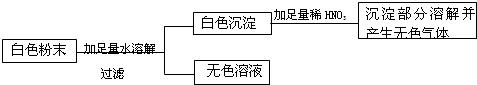
| ¢Ł½«ÉŁŠķ°×É«·ŪÄ©ČÜÓŚĖ®µĆµ½ĪŽÉ«ČÜŅŗA | °×É«·ŪÄ©ÖŠŅ»¶Øƻӊ |
| ¢ŚŌŚAÖŠ¼ÓČė×ćĮæĻõĖį±µČÜŅŗ£¬Éś³É°×É«³ĮµķB£¬¹żĀĖµĆĀĖŅŗC ¢ŪŌŚ°×É«³ĮµķBÖŠ¼ÓČė×ćĮæĻ”ĻõĖį£¬ ¢ÜŌŚĀĖŅŗCÖŠ¼ÓČėĻõĖįŅųČÜŅŗ£¬³öĻÖ°×É«³Įµķ£¬ŌŁµĪ¼ÓĻ”ĻõĖį£¬³Įµķ²»Čܽā£® |
°×É«·ŪÄ©ÖŠŅ»¶ØÓŠ Ģ¼ĖįÄĘŗĶĀČ»ÆÄĘ |
²éæ““š°øŗĶ½āĪö>>
æĘÄæ£ŗ³õÖŠ»Æѧ Ą“Ō“£ŗ ĢāŠĶ£ŗ
²éæ““š°øŗĶ½āĪö>>
æĘÄæ£ŗ³õÖŠ»Æѧ Ą“Ō“£ŗ ĢāŠĶ£ŗ
 £Ø2013?³¤ÄžĒų¶žÄ££©ÓŠŅ»°ü°×É«·ŪÄ©£¬æÉÄÜŗ¬ÓŠK2SO4”¢Na2CO3”¢BaCl2”¢CuSO4ÖŠµÄŅ»ÖÖ»ņ¼øÖÖ£®Č”ŃłČÜÓŚ×ćĮæµÄĖ®£¬ÓŠ°×É«³Įµķ²śÉś£¬¹żĀĖ£¬ĀĖŅŗ³ŹĪŽÉ«£»Ļņ³ĮµķÖŠµĪ¼ÓĻ”ŃĪĖį£¬³ĮµķµÄÖŹĮæÓė¼ÓČėŃĪĖįĢå»żµÄ¹ŲĻµČēĶ¼ĖłŹ¾£®ÓÉ“ĖĶʶĻ°×É«·ŪÄ©µÄ³É·ÖŹĒ£Ø””””£©
£Ø2013?³¤ÄžĒų¶žÄ££©ÓŠŅ»°ü°×É«·ŪÄ©£¬æÉÄÜŗ¬ÓŠK2SO4”¢Na2CO3”¢BaCl2”¢CuSO4ÖŠµÄŅ»ÖÖ»ņ¼øÖÖ£®Č”ŃłČÜÓŚ×ćĮæµÄĖ®£¬ÓŠ°×É«³Įµķ²śÉś£¬¹żĀĖ£¬ĀĖŅŗ³ŹĪŽÉ«£»Ļņ³ĮµķÖŠµĪ¼ÓĻ”ŃĪĖį£¬³ĮµķµÄÖŹĮæÓė¼ÓČėŃĪĖįĢå»żµÄ¹ŲĻµČēĶ¼ĖłŹ¾£®ÓÉ“ĖĶʶĻ°×É«·ŪÄ©µÄ³É·ÖŹĒ£Ø””””£©²éæ““š°øŗĶ½āĪö>>
°Ł¶ČÖĀŠÅ - Į·Ļ°²įĮŠ±ķ - ŹŌĢāĮŠ±ķ
ŗž±±Ź”»„ĮŖĶųĪ„·ØŗĶ²»Į¼ŠÅĻ¢¾Ł±ØĘ½ĢØ | ĶųÉĻÓŠŗ¦ŠÅĻ¢¾Ł±Ø×ØĒų | µēŠÅÕ©Ę¾Ł±Ø×ØĒų | É꥜Ź·ŠéĪŽÖ÷ŅåÓŠŗ¦ŠÅĻ¢¾Ł±Ø×ØĒų | ÉęĘóĒÖČؾŁ±Ø×ØĒų
Ī„·ØŗĶ²»Į¼ŠÅĻ¢¾Ł±Øµē»°£ŗ027-86699610 ¾Ł±ØÓŹĻä£ŗ58377363@163.com