
��6�֣�ȼ������ᷢչ���������ŷdz���Ҫ�����á�
��1�����������䡱�����ѻݼ�ǧ����
����Ȼ������Ҫ�ɷ��Ǽ��飬ʹ����Ȼ����ȼ���ܷ��������ЧӦ�ķ���? (��ܡ����ܡ�)�������ǣ� ��
�� ͬ�¡�ͬѹ�£���ͬ����IJ�ͬ���������ͬ�ķ�������ԭ����ú��Ϊȼ�ϵļ�ͥ��Ҫ������Ȼ����ȼ�ϣ���ߵĸĽ�����Ϊ:
A�����ٿ����Ľ�������������Ȼ���Ľ����� B���������ߵĽ�����
C����������������������Ȼ���Ľ����� D���������ߵĽ�����
��2��ȼ��й©�����Σ�գ�ȼ����ȫ�Ǽ�ͥ�����ͷ�ȴ��¡�Ϊ�˷�ֹȼ����ɢ������ȼ���м�����������������ζ������(C2H5SH)�������ȼ��ʱ����������̼�����������ˮ������д�����ȼ�յĻ�ѧ��Ӧ����ʽ��
 ��ҵ����ϵ�д�
��ҵ����ϵ�д� ͬ��ѧ��һ�ζ���ϵ�д�
ͬ��ѧ��һ�ζ���ϵ�д� �����ܾ�ϵ�д�
�����ܾ�ϵ�д� ���ƿ�����ϵ�д�
���ƿ�����ϵ�д�
| �꼶 | ���пγ� | �꼶 | ���пγ� |
| ��һ | ��һ��ѿγ��Ƽ��� | ��һ | ��һ��ѿγ��Ƽ��� |
| �߶� | �߶���ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
| ���� | ������ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
��Ŀ�����л�ѧ ��Դ�� ���ͣ�

| ȼ������ | ���� | ���� | ���� | ��ϩ | ��Ȳ |
| ��ѧʽ | CH4 | C2H6 | C3H8 | C2H4 | C2H2 |
| ��������104J/g�� | 5.56 | 5.20 | 5.05 | 5.04 | 5.00 |
| ��Ԫ�ص�����������%�� | 25.0 | 20.0 | 18.2 | 14.3 | 7.7 |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ��Ķ�����
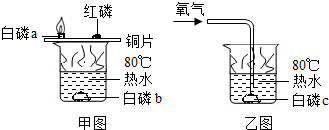
| ||
| ||
| ||
| ||
| ||
| ||
| ||
| ||
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
| ||
| ||
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ��2012��������������п�ģ���Ծ���ѧ�Ծ����壩 ���ͣ������
��6�֣�ȼ������ᷢչ���������ŷdz���Ҫ�����á�
��1�����������䡱�����ѻݼ�ǧ����
����Ȼ������Ҫ�ɷ��Ǽ��飬ʹ����Ȼ����ȼ���ܷ��������ЧӦ�ķ���? (��ܡ����ܡ�)�������ǣ� ��
�� ͬ�¡�ͬѹ�£���ͬ����IJ�ͬ���������ͬ�ķ�������ԭ����ú��Ϊȼ�ϵļ�ͥ��Ҫ������Ȼ����ȼ�ϣ���ߵĸĽ�����Ϊ:
| A�����ٿ����Ľ�������������Ȼ���Ľ����� | B���������ߵĽ����� |
| C����������������������Ȼ���Ľ����� | D���������ߵĽ����� |
�鿴�𰸺ͽ���>>
����ʡ������Υ���Ͳ�����Ϣ�ٱ�ƽ̨ | �����к���Ϣ�ٱ�ר�� | ����թƭ�ٱ�ר�� | ����ʷ���������к���Ϣ�ٱ�ר�� | ������Ȩ�ٱ�ר��
Υ���Ͳ�����Ϣ�ٱ��绰��027-86699610 �ٱ����䣺58377363@163.com