
| ||


 天天向上一本好卷系列答案
天天向上一本好卷系列答案 小学生10分钟应用题系列答案
小学生10分钟应用题系列答案科目:初中化学 来源: 题型:
查看答案和解析>>
科目:初中化学 来源: 题型:
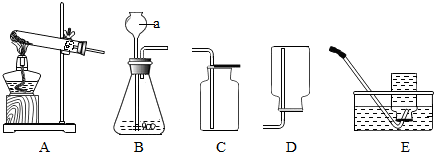
| 实验步骤 | ①称取烧杯的质量 | ②将适量盐酸加入烧杯中并称重 | ③称取少量石灰石样品加入烧杯中,使之与 稀盐酸恰好反应 | ④待反应完全后,称重 |
| 实验图示 |  | |||
| 实验数据 | 烧杯的质量为50.0g | 烧杯和盐酸的质量为100.0g | 石灰石样品样品的质量为12.0g | 烧杯和其中混合物的质量为107.6g |
查看答案和解析>>
科目:初中化学 来源: 题型:
查看答案和解析>>
科目:初中化学 来源: 题型:
| ||
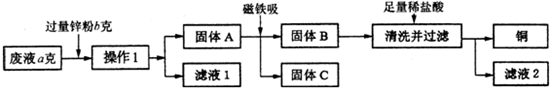
查看答案和解析>>
湖北省互联网违法和不良信息举报平台 | 网上有害信息举报专区 | 电信诈骗举报专区 | 涉历史虚无主义有害信息举报专区 | 涉企侵权举报专区
违法和不良信息举报电话:027-86699610 举报邮箱:58377363@163.com