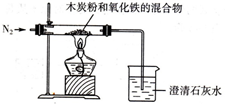
вбжЊФОЬПЗлКЭFe
2O
3ЗДгІЕФЛЏбЇЗНГЬЪНЃК2Fe
2O
3+3C
4Fe+CO
2ЁќЃЌФГЭЌбЇЩшМЦСЫвЛИіЪЕбщЃЌжЄУїбѕЛЏЬњжаКЌгабѕдЊЫиЃЌВЂВтЖЈбѕЛЏЬњжабѕдЊЫиЕФжЪСПЗжЪ§ЃЌЪЕбщзАжУШчЭМЃЎ
ЃЈ1ЃЉЪЕбщЧАЃЌЪзЯШвЊНјааЕФВйзїЪЧ
ЯШЭЈЕЊЦјИЯзпзАжУжаВагрЕФПеЦј
ЯШЭЈЕЊЦјИЯзпзАжУжаВагрЕФПеЦј
ЃЎ
ЃЈ2ЃЉЪЕбщжаЃЌЪЂЗХГЮЧхЪЏЛвЫЎЕФЩеБжаГіЯжЕФЯжЯѓЪЧ
ГЮЧхЪЏЛвЫЎБфЛызЧ
ГЮЧхЪЏЛвЫЎБфЛызЧ
ЃЌдвђЪЧ
ЗДгІЩњГЩСЫДѓСПЕФЖўбѕЛЏЬМ
ЗДгІЩњГЩСЫДѓСПЕФЖўбѕЛЏЬМ
ЃЎ
ЃЈ3ЃЉЪЕбщжаЭЈШыЕФЦјЬхЪЧДПОЛИЩдяЕФN
2ЃЌаДГіВЛгУПеЦјЕФРэгЩЃК
Ђй
ЛьгабѕЦјЃЎШєХіЕНПЩШМадЦјЬхЛсЗЂЩњБЌеЈЃЌгаЮЃЯе
ЛьгабѕЦјЃЎШєХіЕНПЩШМадЦјЬхЛсЗЂЩњБЌеЈЃЌгаЮЃЯе
ЃЛ
Ђк
ПеЦјжаКЌгаКмЖрКЌбѕдЊЫиЕФЮяжЪЃЌетбљЛсИЩШХЖдбѕЛЏЬњжабѕдЊЫиКЌСПЕФХаЖЯ
ПеЦјжаКЌгаКмЖрКЌбѕдЊЫиЕФЮяжЪЃЌетбљЛсИЩШХЖдбѕЛЏЬњжабѕдЊЫиКЌСПЕФХаЖЯ
ЃЛ
вВВЛФмгУГБЪЊЕФЕЊЦјЃЌдвђЪЧ
гАЯьбѕЛЏЬњжабѕдЊЫиКЌСПЕФВтЖЈ
гАЯьбѕЛЏЬњжабѕдЊЫиКЌСПЕФВтЖЈ
ЃЎ
ЃЈ4ЃЉШчгУ3.2gбѕЛЏЬњгыЬМГфЗжЗДгІЃЌВтЕУГЮЧхЪЏЛвЫЎдіжи1.32gЃЌдђбѕЛЏЬњжабѕдЊЫиЕФжЪСПЗжЪ§ЮЊ
30%
30%
ЃЎ

 вбжЊФОЬПЗлКЭFe2O3ЗДгІЕФЛЏбЇЗНГЬЪНЃК2Fe2O3+3C
вбжЊФОЬПЗлКЭFe2O3ЗДгІЕФЛЏбЇЗНГЬЪНЃК2Fe2O3+3C


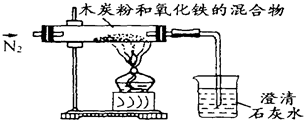 вбжЊФОЬПЗлКЭFe2O3 ЗДгІЕФЛЏбЇЗНГЬЪНЮЊЃК2Fe2O3+3C
вбжЊФОЬПЗлКЭFe2O3 ЗДгІЕФЛЏбЇЗНГЬЪНЮЊЃК2Fe2O3+3C вбжЊФОЬПЗлКЭFe2O3ЗДгІЕФЛЏбЇЗНГЬЪНЃК2Fe2O3+3C
вбжЊФОЬПЗлКЭFe2O3ЗДгІЕФЛЏбЇЗНГЬЪНЃК2Fe2O3+3C 4Fe+CO2ЁќЃЌФГЭЌбЇЩшМЦСЫвЛИіЪЕбщЃЌжЄУїбѕЛЏЬњжаКЌгабѕдЊЫиЃЌВЂВтЖЈбѕЛЏЬњжабѕдЊЫиЕФжЪСПЗжЪ§ЃЌЪЕбщзАжУШчЭМЃЎ
4Fe+CO2ЁќЃЌФГЭЌбЇЩшМЦСЫвЛИіЪЕбщЃЌжЄУїбѕЛЏЬњжаКЌгабѕдЊЫиЃЌВЂВтЖЈбѕЛЏЬњжабѕдЊЫиЕФжЪСПЗжЪ§ЃЌЪЕбщзАжУШчЭМЃЎ 4Fe+CO2ЁќЃЌФГЭЌбЇЩшМЦСЫвЛИіЪЕбщЃЌжЄУїбѕЛЏЬњжаКЌгабѕдЊЫиЃЌВЂВтЖЈбѕЛЏЬњжабѕдЊЫиЕФжЪСПЗжЪ§ЃЌЪЕбщзАжУШчЭМЃЎ
4Fe+CO2ЁќЃЌФГЭЌбЇЩшМЦСЫвЛИіЪЕбщЃЌжЄУїбѕЛЏЬњжаКЌгабѕдЊЫиЃЌВЂВтЖЈбѕЛЏЬњжабѕдЊЫиЕФжЪСПЗжЪ§ЃЌЪЕбщзАжУШчЭМЃЎ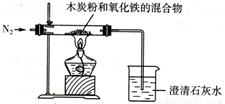
 4Fe+3CO2ЁќЛђепЮЊЃКFe2O3+3C
4Fe+3CO2ЁќЛђепЮЊЃКFe2O3+3C 2Fe+3COЁќ
2Fe+3COЁќ 4FeOЪЎCO2ЁќЃЌЮЊжЦЕУетжжЛюадзюИпЕФДпЛЏМСЃЌгІЯђ480g Fe2O3ЗлФЉжаМгШыЬМЖрЩйПЫЃП ЃЎ
4FeOЪЎCO2ЁќЃЌЮЊжЦЕУетжжЛюадзюИпЕФДпЛЏМСЃЌгІЯђ480g Fe2O3ЗлФЉжаМгШыЬМЖрЩйПЫЃП ЃЎ