
| ʵ����� | ʵ��1 | ʵ��2 | ʵ��3 |
| Cװ���г���������g�� | 3.93 | 3.94 | 3.95 |
| 44 |
| x |
| 197 |
| 3.94g |
| 100 |
| x |
| 44 |
| 0.88g |


 ��������һ���þ�ϵ�д�
��������һ���þ�ϵ�д� Сѧ��10����Ӧ����ϵ�д�
Сѧ��10����Ӧ����ϵ�д�
| �꼶 | ���пγ� | �꼶 | ���пγ� |
| ��һ | ��һ��ѿγ��Ƽ��� | ��һ | ��һ��ѿγ��Ƽ��� |
| �߶� | �߶���ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
| ���� | ������ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
��Ӧ�����ǵ��ճ���������������еĹ�ϵ��
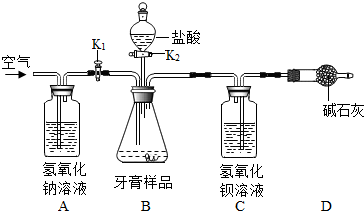
�������˵�θ����_________�����θҺ���ڹ��������θ�������ú�������������ҩ
��ɻ��ⲡʹ�������ƵĻ�ѧԭ��Ϊ���û�ѧ����ʽ��ʾ��_________________________��
�� ����ͷ��������ʱ�������������л��ᣬ����ͷǰ��ӽ������Ĵ����С�մ��
�������ʣ���������ò�����__________���壨�ѧʽ����ʹ��������ͷ���ɶ�ף�������ļ������ʹ��࣬�������Ż������еμ�����____________�����Ե�ζ�ϣ�������������ͷ���ɬ����ɫ���ơ�
�� δ�����ˮ��������ɬζ��������Ϊˮ���ﺬ�����ᡣ��ɬ�ķ���֮һ�ǣ�����
������ˮ����ʯ�һ���ʯ���飬�ñ仯�Ļ�ѧ����ʽ�� ___________________________��Ȼ���������ˮϡ�ͣ�ȡ�ϲ���ҹ������ˮ��5��6�켴�ɳ�ȥɬζ��
�� �˱����涣ҧ��Ƥ���������� ����������������Ƥ����ע���������������¡��������ұ����е������Ʒ________��______�����ţ�Ϳ�ڱ�ҧ��Ƥ���ϣ�ʹ�������ʧ��
| ���� | A | B | C | D |
| ����ˮ | ʳ�� | ���� | ʳ��ˮ | |
| pH | 10 | 3 | 9 | 7 |
�� �����پٳ�һ�������������г�����������������____________________��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ��2007-2008ѧ���꼪��ʡ��������ͨ��ѧ�������꼶���£����л�ѧ�Ծ��������棩 ���ͣ�ѡ����
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ���ѡ��
�鿴�𰸺ͽ���>>
����ʡ������Υ���Ͳ�����Ϣ�ٱ�ƽ̨ | �����к���Ϣ�ٱ�ר�� | ����թƭ�ٱ�ר�� | ����ʷ���������к���Ϣ�ٱ�ר�� | ������Ȩ�ٱ�ר��
Υ���Ͳ�����Ϣ�ٱ��绰��027-86699610 �ٱ����䣺58377363@163.com