
��ѧ��ȤС���ͬѧΪ�˲ⶨij������ʯ��������������������
��ͬѧȡһ�������ij�������������ľ̿�ۻ�Ϻ�����ͼ��ʾװ���Ժ����IJ�������ⶨ����������ʼ�ղ������仯������Ӧ���ɵ������Ͷ�����̼���Լ�������һ����̼��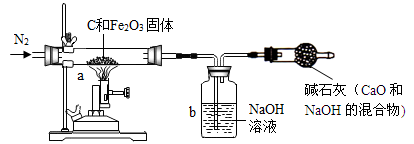
��1�� ʵ���г���ͨ�����ĵ���������ǰ����ͨ��һ��ʱ�䣬�������� ��
��2����Ӧ������ͬѧϨ������ƵĻ��������ֹͣͨ��N2����������������ĺ��
�� ��
��3�����bװ��������������Һ�Զ�����̼����������ȫ�ģ���ô��ͬѧ��bװ��������
������Һ�������仯�ⶨ�������������������� ��ѡ�ƫ����ƫС��
��ȷ������
����ͬѧȡ����������������Ϊ80%�ij������10g����������ϡ���ᣬǡ����ȫ��Ӧ��
����ȥϡ����154.5g���������ʼȲ�����ˮҲ�����ᷢ����Ӧ����
�Լ��㷴Ӧ����Һ�����ʵ���������������д��������̣�
��1����װ���ڵĿ����ž���2��b�е�NaOH��Һ�ᵹ����a�����Ӳ�ʲ�����
���ѣ������ɵ������±������� ��3��ƫС
���������������1����һ����̼��ԭ��������Ҫ�Ȱ�װ���ڵĿ����ž���Ŀ���Ƿ�ֹ��ը��
��2����Ӧ������Ҫ���Թ���ȴ����ֹͣͨ������Ŀ���Ƿ�ֹ���ɵ����ɵ������±�������
��3������̼����������ӦҲ���ܲ���һ����̼,���ᱻ���գ��ʲⶨ��������������������ƫС��
���û�ѧ����ʽ���������Ȼ����������ȿ�����Ȼ�����������
�⣺�跴Ӧ����FeCl3����Ϊx��
Fe2O3+6HCl�T2FeCl3+3H2O
160 325
10g��80% x
X=(325��8g)/160=16.25g
��Һ��FeCl3����������=16.25g/(154.5g+8g)��100%=10%
�𣺷�Ӧ����Һ���Ȼ��������������ֱ���10%
���㣺���û�ѧ����ʽ�ļ��㡢�й��������������ļ���



| �꼶 | ���пγ� | �꼶 | ���пγ� |
| ��һ | ��һ��ѿγ��Ƽ��� | ��һ | ��һ��ѿγ��Ƽ��� |
| �߶� | �߶���ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
| ���� | ������ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
��Ŀ�����л�ѧ ��Դ�� ���ͣ�������
��50g Na2CO3��Һ����μ���һ����������������CaCl2��Һ��ʵ������У����ɳ������������CaCl2��Һ��������ϵ����ͼ��ʾ���Լ��㣺
��1��ǡ����ȫ��Ӧʱ�����ɳ���������Ϊ______________g��
��2������ǡ����ȫ��Ӧʱ��������Һ�����ʵ�����������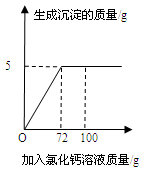
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�������
(7��)ˮ������֮Դ����������ϧˮ��Դ��ÿ����������Ρ�
��1����Ȼ���е�ˮ���Ǵ�ˮ�������ó����� ������������ȷ������Ծ���ˮ��
��2��ˮ���������Ƹ�����Һ��������������ˮ����Һ�¶��������ߵ��� ��
| A���������� | B������� | C������� | D��Ũ���� |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�������
����7�֣�ij������Ʒ�л����������Ȼ��ƣ�Ϊ�˲ⶨ����Ʒ��̼���Ƶ�����������С��ͬѧȷ��ȡ 12.0g��Ʒ����������ʵ�飺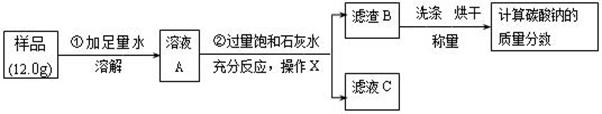
��ش��������⣺
��1������X�õ��IJ����������ձ����������⣬�������� ��
��2����Һ C�е������� ��������Һ C�еμ�ϡ���ᣬд�������з�����һ���кͷ�Ӧ�Ļ�ѧ����ʽ ��
��3����������Bϴ�Ӻ�ɺ���������Ϊ 10.0g , ���㴿����Ʒ�е� Na2CO3�����������Ƕ��٣����������һλС����Ҫ��д��������̣�
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�������
��5�֣�ijУ����С���ͬѧ�ڲⶨ��MgCl2��NaCl��ɵĹ�����������ʱ������������ʵ�顣ȡ20�˹������200����Һ��ƽ���ֳ��ķݣ�Ȼ��ֱ����һ������������NaOH��Һ������ʵ�����ݼ��±���
| | ʵ��һ | ʵ��� | ʵ���� | ʵ���� |
| ����������Һ������/g | 50 | 50 | 50 | 50 |
| ����NaOH��Һ������/g | 10 | 20 | 30 | 40 |
| ���ɳ���������/g | 1 | m | 2.9 | 2.9 |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�������
(6��)����ʱ��ͬѧ�Dz����������ַ����ⶨij�Ȼ�����Һ����������������
��1������ѧ��������һ�����Ȼ�����Һ�м���������������Һ���õ�2.87g�Ȼ������壬����Ȼ�����Һ���Ȼ��Ƶ�����Ϊ���٣������ݻ�ѧ����ʽ��ʽ���㣩
�����ʵ��ⶨ������Һ��������������Ϊ10%��
��2��������������ȡһ��������Һ��������������ʵ���������£�
| �������������g�� | 25.0 |
| ������+ʳ����Һ��g�� | 45.0 |
| ������+ʳ�ξ��壨g�� | 27.4 |
| ���ݴ��� | ��Һ��������������Ϊ |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�������
ij��˾�������Ĵ����Ʒ�о����ֻ�����Ȼ������ʡ�Ϊ�ⶨ��Ʒ��̼���Ƶ�����������20��ʱ����ȡ�ò�Ʒ��Ʒ26��5g�����뵽ʢ��һ������ϡ������ձ��У�̼������ϡ����ǡ����ȫ��Ӧ��������ȫ�ݳ����õ�������NaCl��Һ����Ӧ�����þ�����������ձ��ڻ�����������m���뷴Ӧʱ�䣨t����ϵ����ͼ��ʾ��
��1������CO2��������
��2����Ӧ��������Һ��NaCl������������
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�������
(3��)ʵ������һƿ�����Һ����ʦ��С��ͬѧ��Ʒ����ⶨ�÷�Һ�����������������С��ͬѧ��ȡһ�ྻС�ձ�����������Ϊ18.2g��Ȼ�������е��������������Һ�������������Ϊ33.2g��֮��һö����Ϊ10.8g������������ɰֽ��ĥȥ�����⣩�����С�ձ��з�Ӧ������Ӧ�����Һ������ʱ�ٴγ�����������Ϊ43.9 g����ش��������⣺
��Ӧ�в���������������ǡ�
����÷�Һ�����������������д��������̣�����������һλС������
�������������δ�������Լ�������Ӱ���� (ѡ�ƫ����ƫС��������Ӱ�족) ��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�������
�����ȡ����ij�֡������Ʒ�����������ʽ����Ȼ��ơ�ȷ��ȡ��Ʒ20�ˣ�����100��ϡ������Һʹ��ǡ����ȫ��Ӧ������Ӧ���ʣ�����ʳ���������Ϊ117.8�ˡ�
��1�����ɶ�����̼������ �ˡ�
��2������ϡ��������������Ƕ��٣�
�鿴�𰸺ͽ���>>
����ʡ������Υ���Ͳ�����Ϣ�ٱ�ƽ̨ | �����к���Ϣ�ٱ�ר�� | ����թƭ�ٱ�ר�� | ����ʷ���������к���Ϣ�ٱ�ר�� | ������Ȩ�ٱ�ר��
Υ���Ͳ�����Ϣ�ٱ��绰��027-86699610 �ٱ����䣺58377363@163.com