
�������ƻ���ڹ����ϲ����˸�Ʒ�ʵ����Ͻ���Ǻ��п��ܼ���ƣ���������в��ϻش����⣮
(1)���Ͻ�����е�����ͨ������ʯ(��ѧʽΪNa3AlF6)��ȡ�ģ����з�Ԫ�صĻ��ϼ�Ϊ________��
(2)���ƻ�����õ�ȼ���DZ���(��ѧʽΪC3H8)���������(CH4)������(C4H10)����ͬһϵ�е��л��������������ϵ���л�������(C5H12)������(C6H14)���������ʣ������������������Ļ�ѧʽ�����ƣ���֪�����к�����̼ԭ�ӵ������Ļ�ѧʽ��________�����������к�������ߵ�������________��

| �꼶 | ���пγ� | �꼶 | ���пγ� |
| ��һ | ��һ��ѿγ��Ƽ��� | ��һ | ��һ��ѿγ��Ƽ��� |
| �߶� | �߶���ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
| ���� | ������ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
 2008��5��26�����磬��������ʥ�������д��ݣ��������ƻ���ڹ����ϲ����˸�Ʒ�ʵ����Ͻ���Ǻ��п��ܼ���ƣ���������в��ϣ��ش��й����⣺
2008��5��26�����磬��������ʥ�������д��ݣ��������ƻ���ڹ����ϲ����˸�Ʒ�ʵ����Ͻ���Ǻ��п��ܼ���ƣ���������в��ϣ��ش��й����⣺
| ||
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ������
 2008��5��26�����磬��������ʥ�������д��ݣ��������ƻ���ڹ����ϲ����˸�Ʒ�ʵ����Ͻ���Ǻ��п��ܼ���ƣ���������в��ϣ��ش��й����⣺
2008��5��26�����磬��������ʥ�������д��ݣ��������ƻ���ڹ����ϲ����˸�Ʒ�ʵ����Ͻ���Ǻ��п��ܼ���ƣ���������в��ϣ��ش��й����⣺ 4Al+3O2�������У�����ʯ���ۼ�����ʹAl2O3���������������ӣ�����ʯ�Ļ�ѧʽΪNa3AlF6�������з�Ԫ�صĻ��ϼ�Ϊ________��
4Al+3O2�������У�����ʯ���ۼ�����ʹAl2O3���������������ӣ�����ʯ�Ļ�ѧʽΪNa3AlF6�������з�Ԫ�صĻ��ϼ�Ϊ________���鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
2008��5��26�����磬��������ʥ�������д��ݡ��������ƻ���ڹ����ϲ����˸�Ʒ�ʵ����Ͻ���Ǻ��п��ܼ���ơ���������в��ϣ��ش��й����⣺
��1�����Ͻ�����е�����ͨ�����з�Ӧ�Ƶõģ�2A12O3�����ڣ�![]() 4Al��3O2�������У�����ʯ���ۼ�����ʹAl2O3���������������ӡ�����ʯ�Ļ�ѧʽΪNa3AlF6�������з�Ԫ�صĻ��ϼ�Ϊ________��
4Al��3O2�������У�����ʯ���ۼ�����ʹAl2O3���������������ӡ�����ʯ�Ļ�ѧʽΪNa3AlF6�������з�Ԫ�صĻ��ϼ�Ϊ________��
��2�����ƻ�����õ�ȼ���DZ��飨��ѧʽΪC3H8����������飨CH4�������飨C4H10������ͬһϵ�е��л���D�D����������ϵ���л������飨C5H12�������飨C6H14�����������ʡ������������������Ļ�ѧʽ�����ƣ���֪�����к�����̼ԭ�ӵ������Ļ�ѧʽ��_______
____�����������к�������ߵ�������____________��
��3��������ȫȼ�����ɶ�����̼��ˮ�����仯ѧ��Ӧ����ʽΪ______________________��
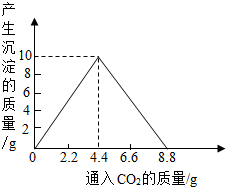
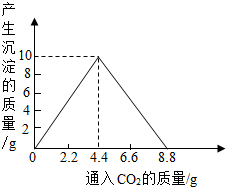
��4������������ȫȼ�պ������CO2���壬ͨ��һ�����ij���ʯ��ˮ�У������ij�����ͨ��CO2�����������ϵ����ͼ��ʾ����������������������________g�������ԭʯ��ˮ���������ʵ�������[��֪��CaCO3+CO2+H2O��Ca(HCO3)2��Ca(HCO3)2������ˮ

�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ��2008�꽭��ʡ̩�����п���ѧ�Ծ��������棩 ���ͣ������
 4Al+3O2�������У�����ʯ���ۼ�����ʹAl2O3���������������ӣ�����ʯ�Ļ�ѧʽΪNa3AlF6�������з�Ԫ�صĻ��ϼ�Ϊ______��
4Al+3O2�������У�����ʯ���ۼ�����ʹAl2O3���������������ӣ�����ʯ�Ļ�ѧʽΪNa3AlF6�������з�Ԫ�صĻ��ϼ�Ϊ______��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�������п����� ���ͣ������
 4Al��3O2�������У�����ʯ���ۼ�����ʹAl2O3���������������ӡ�����ʯ�Ļ�ѧʽΪNa3AlF6�������з�Ԫ�صĻ��ϼ�Ϊ________��
4Al��3O2�������У�����ʯ���ۼ�����ʹAl2O3���������������ӡ�����ʯ�Ļ�ѧʽΪNa3AlF6�������з�Ԫ�صĻ��ϼ�Ϊ________��
�鿴�𰸺ͽ���>>
����ʡ������Υ���Ͳ�����Ϣ�ٱ�ƽ̨ | �����к���Ϣ�ٱ�ר�� | ����թƭ�ٱ�ר�� | ����ʷ���������к���Ϣ�ٱ�ר�� | ������Ȩ�ٱ�ר��
Υ���Ͳ�����Ϣ�ٱ��绰��027-86699610 �ٱ����䣺58377363@163.com