
2011��9�£�ij���౻�غ����°���������������������ߵĽ��ǣ�����ͬѧ�ǿ�չ�˶�����ɷּ����õ�̽���������������ϵ�֪��
��1���ܶ�Ʒ��������С����������������еġ�������ָ
A�������� B����Ԫ�� C����ԭ��
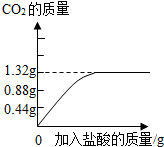
��2������ȥ����Ҫ��������Ħ�����á�ijƷ�������е�Ħ������̼��ƣ�Ϊ�˼��鲢�ⶨ������̼��Ƶ�����������ͬѧ��ȡ��10g���࣬��������ϡ���Ტ���衣ʵ������м�¼�����������γ���ͼ���ߣ�
��Ʒ��������̼��Ƶ���������Ϊ���١�

|
|
|
 |
�١�����¯���з�����Ӧ�Ļ�ѧ����ʽ�ǣ� ���ڡ���Ӧ�ء�����Ҫ������Ӧ�Ļ�ѧ����ʽ�ǣ� ��
�ڡ������ء�����ʯ����ˮ��ַ�Ӧ��ɵõ������dz�ϸС����ʯ�ҽ�����ʯ�ҽ�Ϊ
(ѡ�����Һ������Һ��������Һ��)��
�����˽�����������������CO2�����̼������Һ�������������ŵ��� ��
С���������Ϻ��֪��a��������̼����ͨ������������Һ�������·�Ӧ��
CO2+Ca(OH)2=CaCO3��+H2O�� CaCO3+ H2O + CO2=Ca(HCO3)2��
|
Ca(HCO3)2===== CaCO3��+H2O+ CO2����
Ϊ�˷�ֹ���ø÷��Ƶõ�����̼����л���Ca(HCO3)2������2�б���Ҫ���е�һ�������� ��
��1��B (1��)
��2�� ���Ʒ��������̼��Ƶ�����ΪX
2HCl + CaCO3 = CaCl2 + CO2��+ H2O
100 44
X 1.32g
100:44=X:1.32g
X=3g ��2�֣�
 ���Ʒ��������̼��Ƶ���������Ϊ�� ��1�֣�
���Ʒ��������̼��Ƶ���������Ϊ�� ��1�֣�
��3���� CaCO3![]() CaO+ CO2��(1��)��Na2CO3+Ca(OH)2=CaCO3�� +2NaOH (1��) ��
CaO+ CO2��(1��)��Na2CO3+Ca(OH)2=CaCO3�� +2NaOH (1��) ��
������Һ(1��) �ۿɽ��������ɱ��ͽ��ܼ��� ����1�֣���2�֣�����������Ҳ�ɣ� �������������������Ϊֹ(1��)


 A�ӽ��� ϵ�д�
A�ӽ��� ϵ�д� ȫ�Ų��Ծ�ϵ�д�
ȫ�Ų��Ծ�ϵ�д�
| �꼶 | ���пγ� | �꼶 | ���пγ� |
| ��һ | ��һ��ѿγ��Ƽ��� | ��һ | ��һ��ѿγ��Ƽ��� |
| �߶� | �߶���ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
| ���� | ������ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
��Ŀ�����л�ѧ ��Դ�� ���ͣ��Ķ�����
 ��2012?��������ģ��2011��9�£�ij���౻�غ����°���������������������ߵĽ��ǣ�����ͬѧ�ǿ�չ�˶�����ɷּ����õ�̽���������������ϵ�֪��
��2012?��������ģ��2011��9�£�ij���౻�غ����°���������������������ߵĽ��ǣ�����ͬѧ�ǿ�չ�˶�����ɷּ����õ�̽���������������ϵ�֪��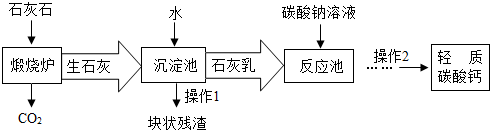
| ||
| ||
| ||
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ��2012�콭��ʡ�Ͼ������������꼶�п���ģ��ѧ�Ծ����������� ���ͣ�������
��(10��)2011��9�£�ij���౻�غ����°���������������������ߵĽ��ǣ�����ͬѧ�ǿ�չ�˶�����ɷּ����õ�̽���������������ϵ�֪��
��1���ܶ�Ʒ��������С����������������еġ�������ָ
A�������� B����Ԫ�� C����ԭ��
��2������ȥ����Ҫ��������Ħ�����á�ijƷ�������е�Ħ������̼��ƣ�Ϊ�˼��鲢�ⶨ������̼��Ƶ�����������ͬѧ��ȡ��10g���࣬��������ϡ���Ტ���衣ʵ������м�¼�����������γ���ͼ���ߣ�
��Ʒ��������̼��Ƶ���������Ϊ���١�
��3����������Ħ����������̼��ƿ���ʯ��ʯ���Ʊ�����ҵ����Ҫ�����������£�
�١�����¯���з�����Ӧ�Ļ�ѧ����ʽ�ǣ� ���ڡ���Ӧ�ء�����Ҫ������Ӧ�Ļ�ѧ����ʽ�ǣ� ��
�ڡ������ء�����ʯ����ˮ��ַ�Ӧ��ɵõ������dz�ϸС����ʯ�ҽ�����ʯ�ҽ�Ϊ
(ѡ�����Һ������Һ��������Һ��)��
�����˽�����������������CO2�����̼������Һ�������������ŵ��� ��
С���������Ϻ��֪��a��������̼����ͨ������������Һ�������·�Ӧ�� CO2+Ca(OH)2=CaCO3��+H2O�� CaCO3+ H2O + CO2=Ca(HCO3)2��
|
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ������
 2011��9�£�ij���౻�غ����°���������������������ߵĽ��ǣ�����ͬѧ�ǿ�չ�˶�����ɷּ����õ�̽���������������ϵ�֪��
2011��9�£�ij���౻�غ����°���������������������ߵĽ��ǣ�����ͬѧ�ǿ�չ�˶�����ɷּ����õ�̽���������������ϵ�֪��
 CaCO3��+H2O+CO2����
CaCO3��+H2O+CO2�����鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ��2011-2012ѧ�꽭��ʡ�Ͼ������������꼶�п���ģ��ѧ�Ծ��������棩 ���ͣ�������
��(10��)2011��9�£�ij���౻�غ����°���������������������ߵĽ��ǣ�����ͬѧ�ǿ�չ�˶�����ɷּ����õ�̽���������������ϵ�֪��
��1���ܶ�Ʒ��������С����������������еġ�������ָ
A�������� B����Ԫ�� C����ԭ��
��2������ȥ����Ҫ��������Ħ�����á�ijƷ�������е�Ħ������̼��ƣ�Ϊ�˼��鲢�ⶨ������̼��Ƶ�����������ͬѧ��ȡ��10g���࣬��������ϡ���Ტ���衣ʵ������м�¼�����������γ���ͼ���ߣ�

��Ʒ��������̼��Ƶ���������Ϊ���١�
��3����������Ħ����������̼��ƿ���ʯ��ʯ���Ʊ�����ҵ����Ҫ�����������£�

�١�����¯���з�����Ӧ�Ļ�ѧ����ʽ�ǣ� ���ڡ���Ӧ�ء�����Ҫ������Ӧ�Ļ�ѧ����ʽ�ǣ� ��
�ڡ������ء�����ʯ����ˮ��ַ�Ӧ��ɵõ������dz�ϸС����ʯ�ҽ�����ʯ�ҽ�Ϊ
(ѡ�����Һ������Һ��������Һ��)��
�����˽�����������������CO2�����̼������Һ�������������ŵ��� ��
С���������Ϻ��֪��a��������̼����ͨ������������Һ�������·�Ӧ�� CO2+Ca(OH)2=CaCO3��+H2O�� CaCO3+ H2O + CO2=Ca(HCO3)2��
|
Ϊ�˷�ֹ���ø÷��Ƶõ�����̼����л���Ca(HCO3)2������2�б���Ҫ���е�һ�������� ��
����������1��������������Ԫ����ɵģ������������������еġ�������ָ��Ԫ�ؽ��
��2����ͼ��֪̼��ƺ�������ȫ��Ӧ�����ɵĶ�����̼����Ϊ1.32g�����ݻ�ѧ����ʽ�б���ʽ���̼��Ƶ����������н��
��3������¯����Ҫ��������̼����ڸ����·ֽ�Ϊ�����ƺͶ�����̼�ķ�Ӧ�����ݻ�ѧ����ʽ����д������ȷ��д��
����������Ҫ����������������ˮ��Ӧ�����������Ƶķ�Ӧ�����ݻ�ѧ����ʽ����д������ȷ��д��
��������Һ������Һ�Ķ�����з����ش�
�ö�����̼����̼������Һ���ɴﵽ���������ɱ��ͽ��ܼ��ţ�
�鿴�𰸺ͽ���>>
����ʡ������Υ���Ͳ�����Ϣ�ٱ�ƽ̨ | �����к���Ϣ�ٱ�ר�� | ����թƭ�ٱ�ר�� | ����ʷ���������к���Ϣ�ٱ�ר�� | ������Ȩ�ٱ�ר��
Υ���Ͳ�����Ϣ�ٱ��绰��027-86699610 �ٱ����䣺58377363@163.com