
| ŃĪĖį Ģå»ż£ŗ500mL »ÆѧŹ½£ŗHCl Ļą¶Ō·Ö×ÓÖŹĮæ£ŗ36.5 ĆÜ¶Č£ŗ1.1g/cm3 ÖŹĮæ·ÖŹż£ŗ30% |
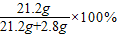 ”Ö88.3%
”Ö88.3%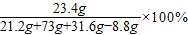 =20%
=20%


| Äź¼¶ | øßÖŠæĪ³Ģ | Äź¼¶ | ³õÖŠæĪ³Ģ |
| øßŅ» | øßŅ»Ćā·ŃæĪ³ĢĶĘ¼ö£” | ³õŅ» | ³õŅ»Ćā·ŃæĪ³ĢĶĘ¼ö£” |
| ø߶ž | ø߶žĆā·ŃæĪ³ĢĶĘ¼ö£” | ³õ¶ž | ³õ¶žĆā·ŃæĪ³ĢĶĘ¼ö£” |
| øßČż | øßČżĆā·ŃæĪ³ĢĶĘ¼ö£” | ³õČż | ³õČżĆā·ŃæĪ³ĢĶĘ¼ö£” |
æĘÄæ£ŗ³õÖŠ»Æѧ Ą“Ō“£ŗ2008ÄźŗŚĮś½Ź”¹ž¶ū±õŹŠÄĻøŚĒųÖŠæ¼»ÆŃ§ČżÄ£ŹŌ¾ķ£Ø½āĪö°ę£© ĢāŠĶ£ŗ½ā“šĢā
 £®
£®
²éæ““š°øŗĶ½āĪö>>
æĘÄæ£ŗ³õÖŠ»Æѧ Ą“Ō“£ŗ2008ÄźŗŚĮś½Ź”¹ž¶ū±õŹŠÄĻøŚĒųÖŠæ¼»ÆŃ§ČżÄ£ŹŌ¾ķ£Ø½āĪö°ę£© ĢāŠĶ£ŗ½ā“šĢā

²éæ““š°øŗĶ½āĪö>>
æĘÄæ£ŗ³õÖŠ»Æѧ Ą“Ō“£ŗ2008ÄźŗŚĮś½Ź”¹ž¶ū±õŹŠÄĻøŚĒųÖŠæ¼»ÆŃ§ČżÄ£ŹŌ¾ķ£Ø½āĪö°ę£© ĢāŠĶ£ŗ½ā“šĢā
| ¾»ŗ¬Įæ£ŗ480g ÓŖŃų³É·Ö£ŗ Ö¬·¾£ŗ20%-25% µ°°×ÖŹ£ŗ25% Šæ£ŗ”Ż20mg Į×£ŗ”Ż500mg øĘ£ŗ”Ż400mg Ģś£ŗ”Ż30mg |
²éæ““š°øŗĶ½āĪö>>
æĘÄæ£ŗ³õÖŠ»Æѧ Ą“Ō“£ŗ2010ÄźÉ½¶«Ź”Ģ©°²ŹŠŠĀĢ©ŹŠµŚŅ»½ĢŃŠĒųÖŠæ¼»ÆŃ§Ä£ÄāŹŌ¾ķ£Ø¶ž£©£Ø½āĪö°ę£© ĢāŠĶ£ŗŃ”ŌńĢā
²éæ““š°øŗĶ½āĪö>>
æĘÄæ£ŗ³õÖŠ»Æѧ Ą“Ō“£ŗ2010ÄźÖŠæ¼Ä£Äā×ØĢā»ć±ą£ŗČĻŹ¶»ÆѧŌŖĖŲ£Ø½āĪö°ę£© ĢāŠĶ£ŗŃ”ŌńĢā
²éæ““š°øŗĶ½āĪö>>
°Ł¶ČÖĀŠÅ - Į·Ļ°²įĮŠ±ķ - ŹŌĢāĮŠ±ķ
ŗž±±Ź”»„ĮŖĶųĪ„·ØŗĶ²»Į¼ŠÅĻ¢¾Ł±ØĘ½ĢØ | ĶųÉĻÓŠŗ¦ŠÅĻ¢¾Ł±Ø×ØĒų | µēŠÅÕ©Ę¾Ł±Ø×ØĒų | É꥜Ź·ŠéĪŽÖ÷ŅåÓŠŗ¦ŠÅĻ¢¾Ł±Ø×ØĒų | ÉęĘóĒÖČؾŁ±Ø×ØĒų
Ī„·ØŗĶ²»Į¼ŠÅĻ¢¾Ł±Øµē»°£ŗ027-86699610 ¾Ł±ØÓŹĻä£ŗ58377363@163.com