
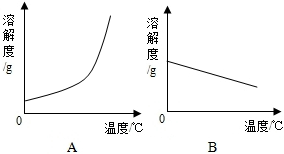
| �¶�/�� | 0 | 20 | 40 | 60 | 80 | 100 | |
| �ܽ��/g | Ca��OH��2 | 0.19 | 0.17 | 0.14 | 0.12 | 0.09 | 0.08 |
| NaOH | 31 | 91 | 111 | 129 | 313 | 336 | |
���� ��1����ͼ����֪��NaOH���ܽ�����¶ȵ����߶����ݴ˷����жϣ�
��2����������Һ��Ϊ������Һ��һ�㷽���ǣ��������ʣ������ܼ��������¶ȣ������Ca��OH��2���ܽ�����¶ȱ仯����������
��3������20��ʱNaOH���ܽ�ȷ����ش�
��4��CaO��ˮ��Ӧ�����������ƣ��ų���������Ca��OH��2���ܽ�����¶ȵ����߶���С������ϱ�����Һ���ʵ�������������ʽ�������
��5���������ƹ������ʵ��ܽ�����¶����߶������������Ƶ��ܽ�����¶ȵ����߶����٣������Ҫ�õ��ϴ�����NaOH���壬Ӧ��ȡ�����������ǽ��½ᾧ�����ˣ�
��6������pHֵ�IJⶨ����������
��� �⣺��1����ͼ����֪��NaOH���ܽ�����¶ȵ����߶����������ܱ�ʾNaOH�ܽ�����ߵ���A��
��2��Ca��OH��2���ܽ�����¶ȵ����߶���С������Ҫʹ�䲻������Һ��Ϊ������Һ�ɲ�ȡ�������ʡ������ܼ������µķ�������ʯ������ˮ��ˮ��Ӧ�ҷ���ʹ��Һ�¶����ߣ����ܽ�ȼ�С��Ҳ��ʹ��������Һ��Ϊ������Һ����ѡD��
��3��20��ʱNaOH���ܽ����91g����10gˮ����ܽ�9.1g������191g ����NaOH��Һ������10gˮ���ٽ��µ�20�棬������NaOH����9.1g��
��4��CaO��ˮ��Ӧ�����������ƣ��ų���������Ca��OH��2���ܽ�����¶ȵ����߶���С��������Һ���ʵ�������������ʽ$\frac{�ܽ��}{�ܽ��+100g}$��100%�����ܽ��Խ����������Ҳ��Խ�����Լ���һ����CaO��õ�����Һ������Һ�����ܽ��С�ڼ���Һ����ʱ��Һ������������ϵ���ң��ף�
��5����Ϊ�������ƹ������ʵ��ܽ�����¶����߶������������Ƶ��ܽ�����¶ȵ����߶����٣������Ҫ�õ��ϴ�����NaOH���壬Ӧ��ȡ�����������ǽ��½ᾧ�����ˣ�
��6��pH��ֽ��ˮ��ʪ��ʹ���ϵ�����������Һ��ϡ�����Ա���������pHֵƫС��
�ʴ�Ϊ����1��A����2��D�� ��3��9.1 g����4��������5�����½ᾧ�����ˣ���6��ƫС��
���� �˽�Ca��OH��2��NaOH�ܽ�ȵ��ܽ�����¶ȵı仯������ⶨ��ҺpHֵ����ȷ���������ܾ���ѧ֪ʶ��ȷ������𣬱������ڿ����֪ʶ�����պ�Ӧ�ã�����ѧ��������������������



| �꼶 | ���пγ� | �꼶 | ���пγ� |
| ��һ | ��һ��ѿγ��Ƽ��� | ��һ | ��һ��ѿγ��Ƽ��� |
| �߶� | �߶���ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
| ���� | ������ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
��Ŀ�����л�ѧ ��Դ�� ���ͣ������
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�ѡ����
| ���� | X | O2 | CO2 | H2O |
| ��Ӧǰ������/g | 23 | 60 | 44 | 0 |
| ��Ӧ�������/g | ���� | 12 | 88 | 27 |
| A�� | ��Ӧ��X������Ϊ0g | |
| B�� | �÷�Ӧ���ɵĶ�����̼��ˮ��������Ϊ88��27 | |
| C�� | ����X�Ļ�ѧʽһ��ΪC2H6O | |
| D�� | �÷�Ӧ�����û���Ӧ |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�ѡ����
| A�� | ��δ����ĥ��Al������CuSO4��Һ�С��Ƚ�Al��Cu�Ļ����� | |
| B�� | ��������ƽ��ҩƷ����ʱ������ֱ�ӽ�ҩƷ������ƽ������ | |
| C�� | ��U��ѹǿ�Ʋ�Һ��ѹǿʱ��Ҫ�����ְ�ѹ̽ͷ�ϵ���ƤĤ���Լ�������� | |
| D�� | ����ԭ������ʵ��ʱ��Ӧ��ͨһ���һ����̼��Ȼ����м��� |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ������
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ���ѡ��
| A�� | ҩ�� | B�� | ��ƿ | C�� | ����©�� | D�� | ����ǯ |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�ʵ��̽����
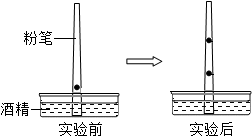 �����ھ�һֻ�۱ʵĴֶ�2cm������һ����īˮ�����۱ʲ���ʢ�оƾ���Һ���������У�����һ��ʱ�䣬���������žƾ�����������īˮҲ�ڷ۱��ϲ����ƶ��������īˮ�ڷ۱��Ϸ�Ϊ���������ϰ벿������ɫ���°벿������ɫ����ͼ��ʾ����������������ش��������⣺
�����ھ�һֻ�۱ʵĴֶ�2cm������һ����īˮ�����۱ʲ���ʢ�оƾ���Һ���������У�����һ��ʱ�䣬���������žƾ�����������īˮҲ�ڷ۱��ϲ����ƶ��������īˮ�ڷ۱��Ϸ�Ϊ���������ϰ벿������ɫ���°벿������ɫ����ͼ��ʾ����������������ش��������⣺�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�ѡ����
| A�� |  ���������� | B�� |  Ϩ��ƾ��� | C�� |  ��ȡҺ�� | D�� |  ���������� |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�ʵ��̽����
�鿴�𰸺ͽ���>>
����ʡ������Υ���Ͳ�����Ϣ�ٱ�ƽ̨ | �����к���Ϣ�ٱ�ר�� | ����թƭ�ٱ�ר�� | ����ʷ���������к���Ϣ�ٱ�ר�� | ������Ȩ�ٱ�ר��
Υ���Ͳ�����Ϣ�ٱ��绰��027-86699610 �ٱ����䣺58377363@163.com