
�Ȼ�����Һ�У����������Ȼ����������������ʣ�����������ʵ�������Ϊ13��10��������������ͬʱ��ȥ���õ��������Ȼ�����Һ������ѡ�õ��Լ������
[����]
A�������������Һ�����к����ʵ�������Ϊ73��98
B���������������Һ�����к����ʵ�������Ϊ73��142
C�����������þ��Һ�����к����ʵ�������Ϊ49��60
D���������������Һ�����к����ʵ�������Ϊ98��71
 �Ķ��쳵ϵ�д�
�Ķ��쳵ϵ�д�
| �꼶 | ���пγ� | �꼶 | ���пγ� |
| ��һ | ��һ��ѿγ��Ƽ��� | ��һ | ��һ��ѿγ��Ƽ��� |
| �߶� | �߶���ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
| ���� | ������ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
��Ŀ�����л�ѧ ��Դ�� ���ͣ��Ķ�����
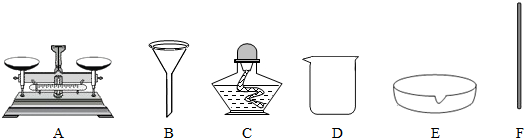
| ���þ�������� | ��ȡ��Ʒ������ |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�

�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
| ||
| ||
| 1g������ȫȼ�ղ���CO2������ | 1g������ȫȼ�շų������� | |
| CH4 | m�T 2.75 2.75 |
56KJ |
| C | 3.67g | 32KJ |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
ijУ��ѧ��ȤС���ͬѧ���������ͼ��ʾ�ļ�������������ǶԷ�Ӧ���Һ�ijɷ�չ����̽����
��������⡿��Ӧ��ķ�Һ�к�����Щ�ɷ֣�
��������ʵ�顿ͼII�з�Ӧ�Ļ�ѧ����ʽΪ ���ɴ��Ƴ���Һ��һ�������Ȼ�����Һ�����ܺ���̼������Һ�����ᡣ

ʵ��һ��ȷ���÷�Һ���Ƿ�������
��1��ѡ���Լ�����������Ļ�ѧ���ʣ�ͬѧ��ѡ������ͼ��ʾ���ֲ�ͬ�������ʣ����У�X������ ����һ�־������ʵ����ƻ�ѧʽ����

��2��ʵ����֤��ijͬѧȡ������Һ���Թ��У������м�������п�����۲쵽 ��ȷ����Һ��һ�����������ᡣ
ʵ�����ȷ����Һ���Ƿ���̼����
| ʵ����� | ���� | ���� |
| ȡ������Һ���Թ��У��μ��� ��������������Һ |
| ��Һ�к���̼���� |
ʵ������������Һ�������Ȼ���
���ӷ�Һ�еõ��������Ȼ��ƣ����Һ�м�������� ���پ��������ᾧ���ɵõ��������Ȼ��ƾ��塣
�鿴�𰸺ͽ���>>
����ʡ������Υ���Ͳ�����Ϣ�ٱ�ƽ̨ | �����к���Ϣ�ٱ�ר�� | ����թƭ�ٱ�ר�� | ����ʷ���������к���Ϣ�ٱ�ר�� | ������Ȩ�ٱ�ר��
Υ���Ͳ�����Ϣ�ٱ��绰��027-86699610 �ٱ����䣺58377363@163.com