

| n |
| 4 |
| ||
| n |
| 2 |
| a |
| 9 |
| a |
| 9 |
| a |
| 9 |



| �꼶 | ���пγ� | �꼶 | ���пγ� |
| ��һ | ��һ��ѿγ��Ƽ��� | ��һ | ��һ��ѿγ��Ƽ��� |
| �߶� | �߶���ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
| ���� | ������ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
��Ŀ�����л�ѧ ��Դ��2008��2009ѧ��ڶ�ѧ������������ѧУ���¿�������ѧ���� ���ͣ�022
����ʹ��һ�������ϴ���ɵġ���ɫ��Ⱦ�����ѳ�Ϊһ�����ص�������⣮ijͬѧ����ij�����ϴ�����ɽ��з���̽��(������ʾ������ֻ��C��H����Ԫ��)�����������ͼʵ��װ�ã�ʹ�����������ڴ�����ȼ�գ��۲�ʵ���������й����ݣ�����Ԫ�غ�����
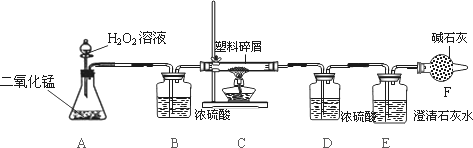
(1)����A���������ķ�Ӧ��ѧ����ʽΪ________��
(2)����B��������________��
(3)����E�������________��
(4)��װ����û����������B����ʹ��������������Ԫ�ص�����������________(�ƫС������ƫ������Ӱ�족��֮һ)��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ������
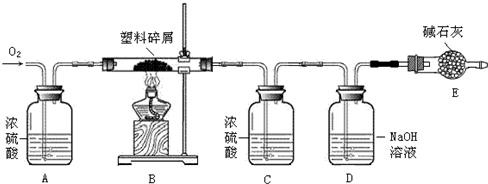
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ��0110 �¿��� ���ͣ�ʵ����

�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ��2009�����ʡ�������п���ѧ�ڶ��ε��п����Ծ��������棩 ���ͣ������
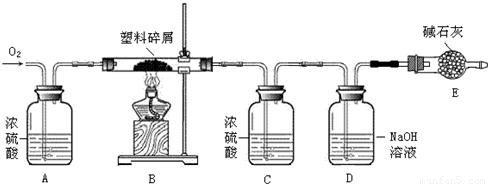
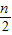 H2O
H2O�鿴�𰸺ͽ���>>
����ʡ������Υ���Ͳ�����Ϣ�ٱ�ƽ̨ | �����к���Ϣ�ٱ�ר�� | ����թƭ�ٱ�ר�� | ����ʷ���������к���Ϣ�ٱ�ר�� | ������Ȩ�ٱ�ר��
Υ���Ͳ�����Ϣ�ٱ��绰��027-86699610 �ٱ����䣺58377363@163.com