
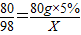 ���X=4.9g
���X=4.9g ×100%=9.8%��
×100%=9.8%�� 


| �꼶 | ���пγ� | �꼶 | ���пγ� |
| ��һ | ��һ��ѿγ��Ƽ��� | ��һ | ��һ��ѿγ��Ƽ��� |
| �߶� | �߶���ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
| ���� | ������ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
 ��2013?��������ģ��ij��ѧѧϰС��ͬѧ��ʵ��������ϰ����һ������������������Һ��
��2013?��������ģ��ij��ѧѧϰС��ͬѧ��ʵ��������ϰ����һ������������������Һ���鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
 ��2013?�껨̨��һģ��ʵ������������õ���������Ϊ10%������������Һ���ܶ�Ϊ1.1g/cm3��������100g��������Ϊ5%������������Һ�����ô���Һ�ⶨij������Һ����������������
��2013?�껨̨��һģ��ʵ������������õ���������Ϊ10%������������Һ���ܶ�Ϊ1.1g/cm3��������100g��������Ϊ5%������������Һ�����ô���Һ�ⶨij������Һ����������������| 80 |
| 98 |
| 80g��5% |
| X |
| 4.9g |
| 50g |
| 80 |
| 98 |
| 80g��5% |
| X |
| 4.9g |
| 50g |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ��2012-2013ѧ�꽭��ʡ�Ͼ����껨̨���п�һģ��ѧ�Ծ��������棩 ���ͣ������
ʵ������������õ���������Ϊ10%������������Һ���ܶ�Ϊ1.1g/cm3��������100g��������Ϊ5%������������Һ�����ô���Һ�ⶨij������Һ����������������
��1������100g��������Ϊ5%������������Һ����Ҫ10%������������Һ�� ��g��
��2����������������Һʱ����Ҫ�IJ����������� �����ιܡ��ձ�����������
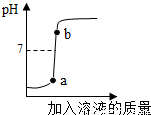
��3������õ�����������Һ�������������������������Һ��Ӧ��ʵ���������Һ��pH�仯������ͼ��ʾ��
�ٸ�����ͼ�仯���ߣ��жϽ��еIJ������� ��������ĸ����
A����������Һ��εμӵ�����������Һ��
B��������������Һ��εμӵ�������Һ��
��b���Ӧ����Һ�е�����Ϊ�� ����д��ѧʽ����
��4������ȫ�к�50gϡ������Һ��ǡ��������õ�����������Һ80g���Լ����ϡ���������������������д��������̣�
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ������
 ij��ѧѧϰС��ͬѧ��ʵ��������ϰ����һ������������������Һ��
ij��ѧѧϰС��ͬѧ��ʵ��������ϰ����һ������������������Һ���鿴�𰸺ͽ���>>
����ʡ������Υ���Ͳ�����Ϣ�ٱ�ƽ̨ | �����к���Ϣ�ٱ�ר�� | ����թƭ�ٱ�ר�� | ����ʷ���������к���Ϣ�ٱ�ר�� | ������Ȩ�ٱ�ר��
Υ���Ͳ�����Ϣ�ٱ��绰��027-86699610 �ٱ����䣺58377363@163.com