
ʵ������һƿ���ܲ������Լ�����ͼ��ʾ�������ȱ�ı�ǩ��ֻʣ�¡�Na���͡�l0%����������֪������ɫҺ�壬�dz��л�ѧ���õ��Լ���
[�������]��ƿ�Լ�������ʲô��Һ�أ�
[��������]���������ǩ������жϣ���ƿ�Լ���������______������ĸ��ţ���
A���� B�� �� C�� ��
[��������]
�ٳ��л�ѧ�����ĺ��ƻ�������NaCl��NaOH��Na2CO3��NHCO3��
��Na2CO3��NaHCO3��Һ���ʼ��ԣ�
�۲ⶨ�����£�20�棩ʱ��4�����ʵ��ܽ�ȵ��������±���ʾ��
��l���ʵ��ܽ��
| ���� | NaCl | NaOH | Na2CO3 | NaHCO3 |
| �ܽ��/g | 36 | 109 | 215 | 9.6 |
[�ó�����]С�������Լ�ƿ��ע��������������10%���ϱ����ܽ�ȵ��������жϣ���ƿ�Լ���������______��
[������ʵ��]�ٿ�����NaOH��Һ���ڿ�����Na2CO3��Һ�� �ۿ�����NaCl��Һ��
��1��Сǿ�ýྻ�IJ�����պȡ����Һ����pH��ֽ�ϣ����pH��7����ƿ�Լ���������______��
��2��СǿΪ��ȷ������Һ�ijɷ֣����ֽ��������2��ʾʵ�飮
��2 ʵ���¼
| �������� | ʵ������ | ���ۼ���ѧ����ʽ |
| ����һ��ȡ��Ʒ���Թ��У��μ� | �������������� | �������ȷ �������ط�Ӧ�Ļ�ѧ���� ʽ��______ |
| ��������Ѳ���������ͨ������ʯ��ˮ�� | ������Һ����� |
[��չ��Ӧ��]��1������ѡ����Сǿ��ͬ���Լ���ȷ������Һ�ijɷ֣���ѡ��______��Һ��
��2��С�մ���Ҫ�ɷ�ΪNaHCO3���г����������Ȼ��ƣ���ѧС���ͬѧΪ�˲ⶨС�մ���NaHCO3����������������������ʵ�飺����Ʒ�����ձ��У������������μ�ϡ���ᣬ�����ٲ�������Ϊֹ����õ��й��������±���ʾ��
| �� �� 1 �� Ʒ | ����ϡ�������� | ��Ӧ����Һ���� |
| ������g�� 9 | 75.4 | 80 |
���㣺��Ӧ��������Һ����������������
�⣺���������ۡ������ǩ�ɼ�����Ϊ��Ԫ�أ����жϸ������ɽ���Ԫ�������ɣ���������������Ԫ�����������ɣ����жϸ����ʲ�����Ϊ�
���ó����ۡ����ݳ�����̼�����Ƶ���ҺΪ9.6g�����жϳ�����̼�����Ƶı�����Һ��������������= ��100%=8.8%��10%����˿��жϸ���Һ������Ϊ̼��������Һ��
��100%=8.8%��10%����˿��жϸ���Һ������Ϊ̼��������Һ��
����Ʋ�ʵ�顿��1�������������ơ�̼������Һ��Ϊ���ԣ���ҺpH������7��ֻ���Ȼ�����Һ��pH=7�����Ը�ƿpH����7����Һ���������Ȼ�����Һ��
��2������̼�����������ᷴӦ�ų�������̼���������������ᷴӦ������������ˣ����ƶϼ���������ʱ��������Լ�Ϊϡ���ᣬ̼���������ᷴӦ�����Ȼ��ơ�ˮ�Ͷ�����̼���۲������ݿ�˵��������Ϊ̼���ƣ��Ѳ�������ͨ�����ʯ��ˮ��ʯ��ˮ�����ǣ���Ӧ�Ļ�ѧ����ʽΪCO2+Ca��OH��2�TCaCO3��+H2O��
����չ��Ӧ�á���1������������������̼���Ʒ�Ӧ���ɰ�ɫ̼��Ƴ����������������Ʋ��ܷ�Ӧ������������ʹ������������Һ���Ȼ�����Һ�ȼ���NaOH��Һ��Na2CO3��Һ��
��2���⣺���������غ㶨�ɵ�CO2������Ϊ��9g+75.4g-80g�T4.4 g
��NaHCO3����ΪX�����ɵ�NaCl������ΪY����
NaHCO3+HCl�TNaCl+H2O+CO2��
84 58.5 44
x y 4.4g =
=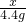 x=8.4 g
x=8.4 g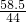 =
=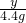 ���� y�T5.85g
���� y�T5.85g
��Ʒ��NaCl������Ϊ��9g-8.4 g�T0.6 g
NaCl����������0.6g+5.85g g�T6.45g
NaCl��Һ��NaCl����������Ϊ��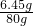 ��100%�T8.1%
��100%�T8.1%
�𣺷�Ӧ��������Һ���Ȼ��Ƶ���������Ϊ��8.1%��
�ʴ�Ϊ��[��������]A
[�ó�����]NaHCO3��Һ
[��������]��1��NaCl��Һ
| �������� | ʵ������ | ���ۼ���ѧ����ʽ |
| ������ϡ���ᣨ��ϡ���ᣩ | ||
Ca��OH��2+CO2=CaCO3��+H2O |
[��չ��Ӧ��]��1��Ca��OH��2/CaCl2�������𰸺���Ҳ�ɣ�
��2���⣺���������غ㶨�ɵ�CO2������Ϊ��9g+75.4g-80g�T4.4 g
��NaHCO3����ΪX�����ɵ�NaCl������ΪY����
NaHCO3+HCl�TNaCl+H2O+CO2��
84 58.5 44
x y 4.4g =
=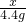 x=8.4 g
x=8.4 g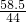 =
=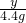 ���� y�T5.85g
���� y�T5.85g
��Ʒ��NaCl������Ϊ��9g-8.4 g�T0.6 g
NaCl����������0.6g+5.85g g�T6.45g
NaCl��Һ��NaCl����������Ϊ��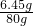 ��100%�T8.1%
��100%�T8.1%
�𣺷�Ӧ��������Һ���Ȼ��Ƶ���������Ϊ��8.1%��


 ��У����ϵ�д�
��У����ϵ�д�
| �꼶 | ���пγ� | �꼶 | ���пγ� |
| ��һ | ��һ��ѿγ��Ƽ��� | ��һ | ��һ��ѿγ��Ƽ��� |
| �߶� | �߶���ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
| ���� | ������ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
 27��ʵ������һƿ���ܲ������Լ�����ͼ�������ȱ�ı�ǩ��ֻʣ�¡�Na���͡�10%����������֪����
27��ʵ������һƿ���ܲ������Լ�����ͼ�������ȱ�ı�ǩ��ֻʣ�¡�Na���͡�10%����������֪����| ���� | NaCl | NaOH | Na2CO3 | NaHCO3 |
| �ܽ��g | 36 | 109 | 215 | 9.6 |
| �������� | ʵ������ | ���ۼ���ѧ����ʽ |
| ȡ�����Թ��У��μ� ������ϡ���� |
�������������� | ����Һ�� ����Һ��̼������Һ ��صĻ�ѧ����ʽ Na2CO3+2HCl�T2NaCl+H2O+CO2�� |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
 ��2012?տ��ģ�⣩ʵ������һƿ���ܲ������Լ�����ͼ�������ȱ�ı�ǩ��ֻʣ�¡�Na���͡�10%����������֪������ɫҺ�壬�dz��л�ѧ���õ��Լ���С����С��ͬѧ�ܸ���Ȥ����������ɷֽ���̽����
��2012?տ��ģ�⣩ʵ������һƿ���ܲ������Լ�����ͼ�������ȱ�ı�ǩ��ֻʣ�¡�Na���͡�10%����������֪������ɫҺ�壬�dz��л�ѧ���õ��Լ���С����С��ͬѧ�ܸ���Ȥ����������ɷֽ���̽����| ���� | NaCl | NaOH | Na2CO3 | NaHCO3 |
| �ܽ��g | 36 | 109 | 215 | 9.6 |
| �������� | ʵ������ | ���ۼ���ѧ����ʽ |
| ȡ�����Թ��У��μ� ����������Һ |
������ɫ�ij��� ������ɫ�ij��� |
�������ȷ ��صĻ�ѧ����ʽ Na2CO3+Ca��OH��2�T2NaOH+CaCO3�� Na2CO3+Ca��OH��2�T2NaOH+CaCO3�� |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ��Ķ�����

| ���� | NaCl | NaOH | Na2C03 | NaHC03 |
| �ܽ��/g | 36 | 109 | 215 | 9.6 |
| �������� | ʵ������ | ���ۼ���ѧ����ʽ |
| ����һ��ȡ��Ʒ���Թ��У��μ� | �������������� | �������ȷ �������ط�Ӧ�Ļ�ѧ���� ʽ�� Ca��OH��2+CO2=CaCO3��+H2O Ca��OH��2+CO2=CaCO3��+H2O |
| ��������Ѳ���������ͨ������ʯ��ˮ�� | ������Һ����� |
| �� �� 1 �� Ʒ | ����ϡ�������� | ��Ӧ����Һ���� |
| ������g�� 9 | 75.4 | 80 |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ��Ķ�����
 ��2011?������ģ��ʵ������һƿ���ܲ������Լ�����ͼ�������ȱ�ı�ǩ��ʣ�¡�Na���͡�10%����������
��2011?������ģ��ʵ������һƿ���ܲ������Լ�����ͼ�������ȱ�ı�ǩ��ʣ�¡�Na���͡�10%����������| ���� | NaCl | NaOH | Na2CO3 | NaHCO3 |
| �ܽ��g | 36 | 109 | 215 | 9.6 |
| �������� | ʵ������ | ���ۼ���ѧ����ʽ |
| ȡ�����Թ��У��μ� ϡ���� ϡ���� |
�������������� | ��صĻ�ѧ����ʽ Na2CO3+2HCl�T2NaCl+H2O+CO2�� Na2CO3+2HCl�T2NaCl+H2O+CO2�� |
| �Ѳ���������ͨ������ʯ��ˮ | ��ɫ���� ��ɫ���� |
�������ȷ |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
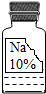 ��2013?������ģ�⣩ʵ������һƿ���ܲ������Լ�����ͼ�������ȱ�ı�ǩ��ֻʣ�¡�Na���͡�10%����������֪������ɫҺ�壬�dz��л�ѧ���õ��Լ���Сǿ��С��ͬѧ�ܸ���Ȥ����������ɷֽ���̽����
��2013?������ģ�⣩ʵ������һƿ���ܲ������Լ�����ͼ�������ȱ�ı�ǩ��ֻʣ�¡�Na���͡�10%����������֪������ɫҺ�壬�dz��л�ѧ���õ��Լ���Сǿ��С��ͬѧ�ܸ���Ȥ����������ɷֽ���̽����| ���� | NaCl | NaOH | Na2CO3 | NaHCO3 |
| �ܽ��g | 36 | 109 | 215 | 9.6 |
| �������� | ʵ������ | ���ۼ���ѧ����ʽ |
| ȡ�����Թ��У��μ� ���ᣨ���ᣩ ���ᣨ���ᣩ |
�������������� | ����Һ�� Na2CO3 Na2CO3 ����Ӧ�Ļ�ѧ����ʽNa2CO3+2HCl=2NaCl+H2O+CO2�� Na2CO3+2HCl=2NaCl+H2O+CO2�� |
�鿴�𰸺ͽ���>>
����ʡ������Υ���Ͳ�����Ϣ�ٱ�ƽ̨ | �����к���Ϣ�ٱ�ר�� | ����թƭ�ٱ�ר�� | ����ʷ���������к���Ϣ�ٱ�ר�� | ������Ȩ�ٱ�ר��
Υ���Ͳ�����Ϣ�ٱ��绰��027-86699610 �ٱ����䣺58377363@163.com