
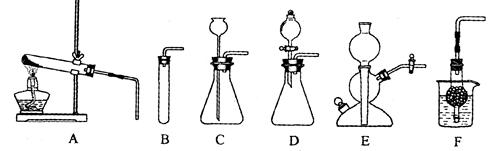
��10����36����ͼA��F��ʵ�����Ʊ�ijЩ���������װ��ʾ��ͼ��
(1)ʵ�����Ʊ�CO2�Ļ�ѧ����ʽΪ��__________________________________ ���õķ���װ���У�______________(ѡ����ͼ��ĸ���)��
(2)װ��E��F�����װ��C�ڲ������������Ϊ��______________________________��
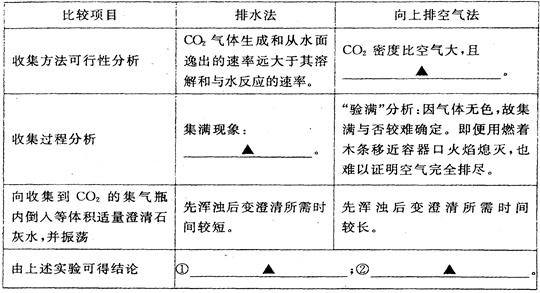
(3)��ˮ���ռ����ſ������ռ�CO2�ıȽ�(�ֱ��������ͬ��2������ƿ�ռ�)
(4)��״ʯ��ʯ������ϡ���ᷴӦ�������ݳ������Ժ�������ҺpH����2����ʱ
ȡ������Ӧ����Һ����ε���̼������Һ������pH����̽����������⣬����������
[pHΪ�����꣬ʱ��s(��)Ϊ������]��
��д��AB�����йػ�ѧ����ʽ____________________________________��
___________________________________________
��д��BC��ƽ̨���λ�ѧ����ʽ___________________________________________
��CD��������ԭ���ǣ�___________________________________________________��
(1) CaCO 3+ 2HCl === CaCl2 +H2 O + CO2�� BCDEF
(2) ���ڿ��Ʒ�Ӧ�ķ�����ֹͣ
(3) ���������Ӧ ����ƿ��Һ���½���ƿ�ڣ��������ݴ�ƿ���ݳ�
���ۢ���ˮ������������̼�������Ի�������㡣
����ˮ���ռ����Ķ�����̼����Ũ�ȻȽϸߡ�
(4) ��Na2CO 3+ 2HCl ===2 NaCl + H2 O + CO2��
CaCO 3+ 2HCl === CaCl2 +H2 O + CO2��
��Na2CO 3+ CaCl2===2NaCl + CaCO 3��
��̼������Һ�ʼ��ԣ�������̼����ʹ��Һ������ǿ
����:��



| �꼶 | ���пγ� | �꼶 | ���пγ� |
| ��һ | ��һ��ѿγ��Ƽ��� | ��һ | ��һ��ѿγ��Ƽ��� |
| �߶� | �߶���ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
| ���� | ������ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
��Ŀ�����л�ѧ ��Դ�� ���ͣ�

�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ��2010��ȫ���п����������ר���� ������Ʊ���һ�� ���ͣ�̽����
��10����36����ͼA��F��ʵ�����Ʊ�ijЩ���������װ��ʾ��ͼ��
(1)ʵ�����Ʊ�CO2�Ļ�ѧ����ʽΪ��__________________________________ ���õķ���װ���У�______________ (ѡ����ͼ��ĸ���)��
(2)װ��E��F�����װ��C�ڲ������������Ϊ��______________________________��
(3)��ˮ���ռ����ſ������ռ�CO2�ıȽ�(�ֱ��������ͬ��2������ƿ�ռ�)
(4)��״ʯ��ʯ������ϡ���ᷴӦ�������ݳ������Ժ�������ҺpH����2����ʱ
ȡ������Ӧ����Һ����ε���̼������Һ������pH����̽����������⣬����������
[pHΪ�����꣬ʱ��s(��)Ϊ������]��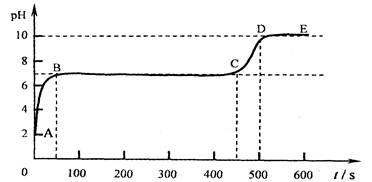
��д��AB�����йػ�ѧ����ʽ____________________________________��
___________________________________________
��д��BC��ƽ̨���λ�ѧ����ʽ___________________________________________
��CD��������ԭ���ǣ�___________________________________________________��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ��2010��ȫ���п����������ר����������Ʊ���һ�� ���ͣ�̽����
��10����36����ͼA��F��ʵ�����Ʊ�ijЩ���������װ��ʾ��ͼ��

(1)ʵ�����Ʊ�CO2�Ļ�ѧ����ʽΪ��__________________________________ ���õķ���װ���У�______________ (ѡ����ͼ��ĸ���)��
(2)װ��E��F�����װ��C�ڲ������������Ϊ��______________________________��
(3)��ˮ���ռ����ſ������ռ�CO2�ıȽ�(�ֱ��������ͬ��2������ƿ�ռ�)

(4)��״ʯ��ʯ������ϡ���ᷴӦ�������ݳ������Ժ�������ҺpH����2����ʱ
ȡ������Ӧ����Һ����ε���̼������Һ������pH����̽����������⣬����������
[pHΪ�����꣬ʱ��s(��)Ϊ������]��
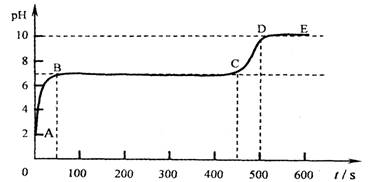
��д��AB�����йػ�ѧ����ʽ____________________________________��
___________________________________________
��д��BC��ƽ̨���λ�ѧ����ʽ___________________________________________
��CD��������ԭ���ǣ�___________________________________________________��
�鿴�𰸺ͽ���>>
����ʡ������Υ���Ͳ�����Ϣ�ٱ�ƽ̨ | �����к���Ϣ�ٱ�ר�� | ����թƭ�ٱ�ר�� | ����ʷ���������к���Ϣ�ٱ�ר�� | ������Ȩ�ٱ�ר��
Υ���Ͳ�����Ϣ�ٱ��绰��027-86699610 �ٱ����䣺58377363@163.com