
| 106 |
| x |
| 171 |
| 17.1g |
| 197 |
| z |
| 80 |
| y |
| 48g |
| 330.9g |


 �¿α�������������ҵ�������γ�����ϵ�д�
�¿α�������������ҵ�������γ�����ϵ�д� ����ν����Ž̲��㽭���̴�ѧ������ϵ�д�
����ν����Ž̲��㽭���̴�ѧ������ϵ�д� �����Ļ������������������ϵ�д�
�����Ļ������������������ϵ�д�
| �꼶 | ���пγ� | �꼶 | ���пγ� |
| ��һ | ��һ��ѿγ��Ƽ��� | ��һ | ��һ��ѿγ��Ƽ��� |
| �߶� | �߶���ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
| ���� | ������ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
| A��CaO |
| B��H2O |
| C��Al2O3 |
| D��Fe3O4 |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
| ��� | �� | �� | �� | �� |
| ���� | ���� | ˮ | ��ʯ�� | ���� |
| ��ѧʽ | CH3COOH | H2O | Ca��OH��2 | Na2CO3 |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
| A��146 | B��92 |
| C��136 | D��238 |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
| A����������ƽ��ȡ5.62g �Ȼ��ƹ��� |
| B����100����Һ����ȡ50.8����Һ�� |
| C����pH��ֽ��������pHֵΪ3 |
| D����5mLˮ��5mL�ƾ����Ƴ�10 mL�ƾ���Һ |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
| A���ƾ��ӷ� | B����˿���� |
| C��ľ��ȼ�� | D���������� |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
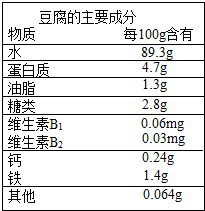 �ƶ������й��Ŵ���һ����Ҫ����������Զ�ľ����������Ե�������ɽˮĥ���Ƴɣ��Գ����ڸ��ۻ�������ӯ�ڶ�����������Ҫ�ɷ�����ͼ��ʾ��
�ƶ������й��Ŵ���һ����Ҫ����������Զ�ľ����������Ե�������ɽˮĥ���Ƴɣ��Գ����ڸ��ۻ�������ӯ�ڶ�����������Ҫ�ɷ�����ͼ��ʾ���鿴�𰸺ͽ���>>
����ʡ������Υ���Ͳ�����Ϣ�ٱ�ƽ̨ | �����к���Ϣ�ٱ�ר�� | ����թƭ�ٱ�ר�� | ����ʷ���������к���Ϣ�ٱ�ר�� | ������Ȩ�ٱ�ר��
Υ���Ͳ�����Ϣ�ٱ��绰��027-86699610 �ٱ����䣺58377363@163.com