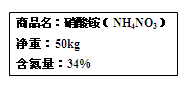
��10�֣�ijѧϰС���ͬѧ��ѧϰ��Na
2CO
3��NaHCO
3�����ʺ��˽���Ƕ��������ᷴӦ����CO
2���壬��ô��μ���Na
2CO
3��NaHCO
3�أ��������������ʣ����ǽ���������̽����
���������ϡ���1��Ca(HCO
3)
2������ˮ��
��2��NaHCO
3�������ȷֽ�����̼���ơ�������̼��ˮ��
���� �롿��1����ͬѧ��Ϊ���ó���ʯ��ˮ����Na
2CO
3��NaHCO
3��Һ��
��2����ͬѧ��Ϊ����CaCl
2��Һ����Na
2CO
3��NaHCO
3��Һ��
�ס�����ͬѧ�IJ��������ݳ���ʯ��ˮ��CaCl
2��Һ�ֱ���Na
2CO
3��Һ��Ӧ���г�����������֪��Ca(HCO
3)
2������ˮ����˲²����ʯ��ˮ��CaCl
2��Һ�ֱ���NaHCO
3��Һ��ϲ�������������Ӷ������������Һ��
��3����ͬѧ��Ϊ����Na
2CO
3��NaHCO
3���ü��ȵķ�������
��ʵ��̽������1����ͬѧ����֧�ֱ�ʢ������Na
2CO
3��NaHCO
3��Һ���Թ��У����������ʯ��ˮ���۲쵽��֧�Թ��е�������ͬ���������˰�ɫ������ʵ��������벻һ�£��������ó���ʯ��ˮ����Na
2CO
3��NaHCO
3��Һ��
��2����ͬѧ��CaCl
2��Һ���뵽�ֱ�ʢ������Na
2CO
3��NaHCO
3��Һ���Թ��У�������֧�Թ���Ҳ�������˰�ɫ������ʵ�����������ϣ������ݹ۲쵽������ʵ����������Ϊ�Կ���CaCl
2��
Һ����Na
2CO
3��NaHCO
3��Һ��
��3����ͬѧ�ֱ�ȡ��һ������Na
2CO
3��NaHCO
3�����ڴ��Թ��м��ȣ���ͼ��ʾ����
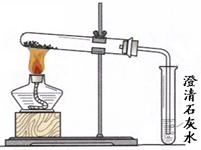
�� ����Na
2CO
3ʱ����ʼ����С�Թ������������ݲ������������ȣ��������٣�δ������ʯ��ˮ����ǣ�
�� ����NaHCO
3ʱ����ͬѧ�۲쵽ʵ��������ٲ�ͬ��֤ʵ���Լ��IJ����Ǻ����ġ�
���������ۡ���1��С��ͬѧ������ʵ��չ�������ۣ��Լ�ͬѧ������ʵ������˱Ƚϣ�������������Һ�н������ͬ���ӵ��۽Ƕȷ�����ԭ����ͼ1��ͼ2������д��Na
2CO
3�����ʯ��ˮ������Ӧ�Ļ�ѧ����ʽ
���NaHCO
3�����ʯ��ˮ���ʱ���뷴Ӧ�����ӣ�
��
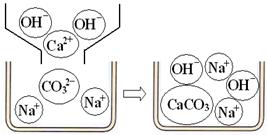
ͼ1������ʯ��ˮ��Na
2CO
3��Һ��Ӧ
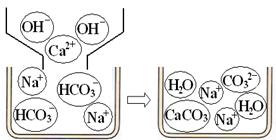
ͼ2����ʯ��ˮ��NaHCO
3��Һ��Ӧ
��2����ͬѧ������ʵ���У���Ҷ�CaCl
2��Na
2CO
3��Һ�ķ�Ӧ�Ƚ���Ϥ������CaCl
2��NaHCO
3��Һ��ϲ����������������⣬ͬѧ���ֽ�һ�����������ϣ��˽CaCl
2��NaHCO
3��Һ�ɷ������·�Ӧ��CaCl
2+2NaHCO
3��CaCO
3��+2NaCl+CO
2��+H
2O,���ͬѧ��֪������ͬѧ��ͨ���۲쵽
����������Na
2CO
3��NaHCO
3��Һ�ġ�
����CaCl
2��NaHCO
3��ҺΪʲô�ܷ���������Ӧ����ʦָ���䷴Ӧԭ���ϸ��ӣ��д��ڽ��ѧϰ�н�һ��̽����
��3����ͬѧ�ڼ���Na
2CO
3����ʱ����ʼ�����������ݵ�ԭ����
�������������Na
2CO
3����
�ֽ⣨��ס����ס������ڼ���NaHCO
3����ʱ����ͬѧ�۲쵽��ʵ��������
����дһ�֣���
����չӦ�á���1������Na
2CO
3�������NaHCO
3����ͨ��
������ȥ��
��2����105g�Ȼ�����Һ��100g5.3%��̼������Һ��ϣ�ǡ����ȫ��Ӧ����Ӧ������Һ�����ʵ����������Ƕ��٣���д�������Ľ�����̣���

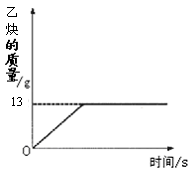
 + C2H2��������X��һ�ּ��X�Ļ�ѧʽΪ ��
+ C2H2��������X��һ�ּ��X�Ļ�ѧʽΪ ��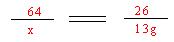 ������1�֣�
������1�֣�


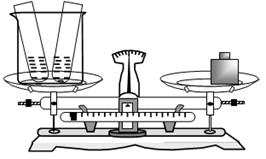



 С�ո��ݡ����Dz�������Ϊ4.3g������õ�����̼��Ƶ���������Ϊ ��
С�ո��ݡ����Dz�������Ϊ4.3g������õ�����̼��Ƶ���������Ϊ ��