
| ָ �� | ����ʵ�� | ����Ԥ�� | ||
| API | ���������ȼ� | API | ���������ȼ� | |
| ������������ | 26 | �� | 20-35 | �� |
| �������� | 38 | �� | 30-45 | �� |
| �������� | 59 | �� | 55-75 | �� |
| ˵ �� | Ԥ�ƽ��տ��������Ϻã�����������Ӱ�죬������������ | |||
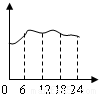
 B��
B��
 D��
D��
 2CO+CO2+6H2O����˿���һ����̼�Ͷ�����̼��������Ϊ2×��12+16������12+16×2��=14��11��
2CO+CO2+6H2O����˿���һ����̼�Ͷ�����̼��������Ϊ2×��12+16������12+16×2��=14��11�� CaO+CO2����CaO+SO2=CaSO3��2CaSO3+O2=2CaSO4��
CaO+CO2����CaO+SO2=CaSO3��2CaSO3+O2=2CaSO4��


| �꼶 | ���пγ� | �꼶 | ���пγ� |
| ��һ | ��һ��ѿγ��Ƽ��� | ��һ | ��һ��ѿγ��Ƽ��� |
| �߶� | �߶���ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
| ���� | ������ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
��Ŀ�����л�ѧ ��Դ��2009�������ʡ�п���ѧģ���Ծ����ģ��������棩 ���ͣ�ѡ����
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ��2011�꽭��ʡ���Ƹ��й������¼���ѧ�п���ѧһģ�Ծ��������棩 ���ͣ�ѡ����
�鿴�𰸺ͽ���>>
����ʡ������Υ���Ͳ�����Ϣ�ٱ�ƽ̨ | �����к���Ϣ�ٱ�ר�� | ����թƭ�ٱ�ר�� | ����ʷ���������к���Ϣ�ٱ�ר�� | ������Ȩ�ٱ�ר��
Υ���Ͳ�����Ϣ�ٱ��绰��027-86699610 �ٱ����䣺58377363@163.com