�⣺��1����ͭ�Ƴɸ��ִ��ߣ��������������õĵ����ԣ��ʴ�Ϊ����
��2�����������ĺϽ𣬲����ǹ����β��ϣ�����̥���ںϳɲ��ϣ���ë������Ȼ���ϣ�
��3���������ķ�Ӧ����������������д�ڵȺŵ�ǰ�ߣ����������Ȼ�����ˮд�ڵȺŵ��ұߣ��ù۲취��ƽ���ɣ����Է���ʽ�ǣ�Fe
2O
3+6HCl�T2FeCl
3+3H
2O
�����ⷴӦ�����������������Ӧ����Ӧ�����������ᣬ���������Ȼ��������������ù۲취��ƽ��������������������ţ����Է���ʽ�ǣ�Fe+2HCl�TFeCl
2+H
2����
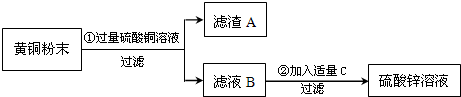
��4��A����ͬ���������ֽ�����������ϡ���ᷴӦʱ��þ����������࣬п�����������٣���A����
B������Fe��Mg��Zn ���ֽ����ֱ��ϡ�����ַ�Ӧ���õ���������������2g��
����뷴Ӧ��Fe������Ϊx��Mg������Ϊy��Zn������Ϊz��
Fe+2HCl=FeCl
2+H
2����Mg+2HCl=MgCl
2+H
2����Zn+2HCl=ZnCl
2+H
2����
56 2 24 2 65 2
x 2g y 2g z 2g
����

��֮�ã�x=56g�����ݣ�
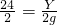
���y=24g������

���z=65g��
��Mg��Zn��Fe��������Ϊ��24g��65g��56g=24��65��56����B��ȷ��
C�����ڲ����������������е������ӣ����ĵ������ͬ������������Ҳ�Dz���ͬ�ģ���C����
D����Ϊ�����������������е������ӣ��ʲ��뷴Ӧ��HCl��������ȣ���������������������ȣ���D��ȷ��
��ѡBD��
�ʴ�Ϊ����1���ڣ���2��A����3��Fe
2O
3+6HCl�T2FeCl
3+3H
2O�� Fe+2HCl�TFeCl
2+H
2������4��BD��
��������1�����ʵĽṹ�������ʵ����ʣ����ʵ����ʾ������ʵ���;����2�����ݺϽ���жϷ������ǣ���3�����ݷ���ʽ����д�������ǣ���4��Fe��Mg��Zn���ֽ����ֱ��ϡ���ᷴӦ�Ļ�ѧ����ʽΪ��Fe+2HCl=FeCl
2+H
2����Mg+2HCl=MgCl
2+H
2����Zn+2HCl=ZnCl
2+H
2�����ɴ˿�֪�������������������е������ӣ���Ҫ�õ���ͬ����������������������ٲ��뷴Ӧ��HCl��������ȣ��ڲ��뷴Ӧ��Fe��Mg��Zn���������ϵõ���ͬ����������������
��������ѡ�����û�ѧ����ʽ�ͼ��跨�������ϼ������û�ѧ����ʽ�������ʱ�����ü��跨���Խ����кܴ����ã�����ѧ���������⡢��������������



 ��֮�ã�x=56g�����ݣ�
��֮�ã�x=56g�����ݣ�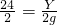 ���y=24g������
���y=24g������ ���z=65g��
���z=65g��

 һŵ��ҵ�����ҵ���ּ�����������������ϵ�д�
һŵ��ҵ�����ҵ���ּ�����������������ϵ�д�