
ijͬѧ��һƿ�治�ƶ����ֱ��ʵ�NaOH��������������̽��������Ϊ�����̽��ʵ�飺
��1��������⣺�������ɵ�������ʲô��
��2�����в��룺���ʺ������ɵ����ʿ������������������� ��д��ѧʽ����
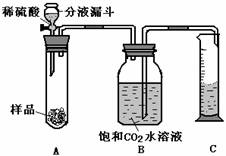
��3�����ʵ�飺���������ͼ��ʾ��һ��װ��������ʵ����֤��ͼ�е�������������ȥ����
��4��ʵ����֤��
�ٰ�ͼ���Ӻ�װ��
����������ƽȷ����2����Ʒ������A�Թ��У���B�е��뱥�͵�CO2��Һ��ƿ������
�۴ӷ�Һ©���м����Թ�����ϡ����رջ�������Ӧ������Ͳ���ռ�����Һ��Ϊ220ml��
a.�ж���Ʒ�ѱ��ʵ���������������������������������
b.�ڢٺ͢�֮��ȱ�ٵ�һ��ʵ�鲽��������������������������
c.B�еı���CO2��Һ������ˮ������������������������������������������������
d.�ж�A�е����ϡH2SO4�ѹ����ı�־����������������������������������������
e.����������Ʒ�б������ɵ����ʵ�����������(CO2�ܶ���1.8g/L����)������������ ��
��5���������ϡ������ܽ��������ȷ�ġ�
��6�����������ۣ�����װ���Dz���������ȷ�ⶨ�ѱ��ʵ���Ʒ��NaOH�����������ģ����������������������������������������� �������������������������� ��
 �Ͻ�ƽСѧ��������ϵ�д�
�Ͻ�ƽСѧ��������ϵ�д�
| �꼶 | ���пγ� | �꼶 | ���пγ� |
| ��һ | ��һ��ѿγ��Ƽ��� | ��һ | ��һ��ѿγ��Ƽ��� |
| �߶� | �߶���ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
| ���� | ������ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
����ʡ������Υ���Ͳ�����Ϣ�ٱ�ƽ̨ | �����к���Ϣ�ٱ�ר�� | ����թƭ�ٱ�ר�� | ����ʷ���������к���Ϣ�ٱ�ר�� | ������Ȩ�ٱ�ר��
Υ���Ͳ�����Ϣ�ٱ��绰��027-86699610 �ٱ����䣺58377363@163.com