

| ʵ����� | ʵ������ | ʵ����� |
| ��a��ȡ������Ʒ���Թ��У���ˮ�ܽ����������Ȼ�����Һ�� | �а�ɫ�������� | ��Ʒ�к���̼���� |
| ��b�����ã�ȡ�ϲ���Һ���Թ��У��μӷ�̪��Һ�� | ��Һ���ɫ | ��Ʒ�к����������� |
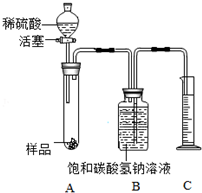
���� ��1������̼���ơ�������������Һ�ж��Լ��ԣ��������������÷�̪����Ҫ�Ƚ�̼���Ƽ��鲢��ȥ�������������������ӽ��з�����
��2�������������̼���ơ��������Ʒ�Ӧ���з�����
��3�������������ƺ����ᷢ�����кͷ�Ӧ��ų�������ʹ����������ͽ��з�����
��4�����ݶ�����̼���ܶȺ���������������̼��������Ȼ���ϻ�ѧ����ʽ�����̼���Ƶ�������
��5�����ݼ����ϡ������ų�һ���������������ʹ����Ķ�����̼���ƫ��̼���Ƶ���������Ҳ��ƫ����з�����
��6�������������Ʊ�������̼���ƣ���ȥ̼���ƿ��Լ�������������������Һ��
��7������̼���ƺ��������Ʒ�Ӧ������̼��Ƴ������������ƽ��з�����
��� �⣺��1��̼���ơ�������������Һ�ж��Լ��ԣ��������������÷�̪����Ҫ�Ƚ�̼���Ƽ��鲢��ȥ�������������������ӣ�����
| ʵ����� | ʵ������ | ʵ����� |
| ��a��ȡ������Ʒ���Թ��У���ˮ�ܽ����������Ȼ�����Һ�� | �а�ɫ�������� | ��Ʒ�к���̼���� |
| ��b�����ã�ȡ�ϲ���Һ���Թ��У��μӷ�̪��Һ�� | ��Һ��ɺ�ɫ | ��Ʒ�к����������� |
| ʵ����� | ʵ������ | ʵ����� |
| �������Ȼ�����Һ | ||
| ��̪��Һ�� | ��Һ��ɺ�ɫ |
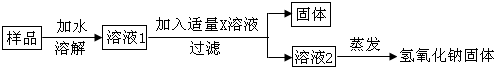
���� �ڽ������ʱ�����ȷ������п�������⣬Ȼ����ѧ����֪ʶ��������������ʾ���н��



| �꼶 | ���пγ� | �꼶 | ���пγ� |
| ��һ | ��һ��ѿγ��Ƽ��� | ��һ | ��һ��ѿγ��Ƽ��� |
| �߶� | �߶���ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
| ���� | ������ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
��Ŀ�����л�ѧ ��Դ�� ���ͣ�ѡ����
| A�� | ����ϡ����ʱ����������Ͳ�м���һ������Ũ���ᣬ������ע��ˮ�����Ͻ��� | |
| B�� | ����ʵ���У����������ڵ�Һ����ȫ���ɺ�����ֹͣ���� | |
| C�� | ��©������Һ��ʱ��Һ���Ը�����ֽ��Ե | |
| D�� | ���װ��������ʱ��Ӧ�Ȱѵ���һ�˷���ˮ�У���˫�ֽ����Թ� |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ������

| ��� | ��Ʒ���� | CO2����� |
| ��1�� | 0.21g | 22.39mL |
| ��2�� | 0.21g | 22.41mL |
| ��3�� | 0.21g | 22.40mL |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ������
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ������
| 1g������ȫȼ�շų���CO2���� | 1g������ȫȼ�շų������� | |
| CH4 | m | 56KJ |
| C | 3.67g | 32kJ |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�ѡ����
| A�� | ��ѧ��Ӧǰ��ԭ�ӵĸ������������ | |
| B�� | �����ڻ�ѧ��Ӧǰ�����������ͻ�ѧ����û�з����仯 | |
| C�� | �������ڻ�����䣬�����������Ŀ�ȼ�� | |
| D�� | ϴ�Ӽ�����ȥ���ۣ�����Ϊ�������黯���� |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ������


�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ������
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ������
�鿴�𰸺ͽ���>>
����ʡ������Υ���Ͳ�����Ϣ�ٱ�ƽ̨ | �����к���Ϣ�ٱ�ר�� | ����թƭ�ٱ�ר�� | ����ʷ���������к���Ϣ�ٱ�ר�� | ������Ȩ�ٱ�ר��
Υ���Ͳ�����Ϣ�ٱ��绰��027-86699610 �ٱ����䣺58377363@163.com