
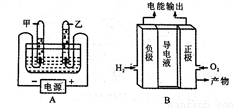
A��J���dz��л�ѧ���õ����ʡ�����B��E��H��J�ǵ��ʣ������Ϊ���������C�����������������ת����ϵ����ͼ��ʾ�����ַ�Ӧ������P��Ӧ��������ȥ����

��ش��������⣺
��д������D��J�Ļ�ѧʽ��D____________,J____________��
�ơ�����C��B����ת�����̣�����Ȼ���г�Ϊ________________���á�
�Ƿ�Ӧ�ٵķ�Ӧ����______________���������Ӧ����________________��
��д����Ӧ�ڵĻ�ѧ����ʽ��______________________________________��
����Ҫʵ�֡�H+I����ɫ����J����ת��������I���������������_________________��
 ����ȫ���ִʾ��ƪ��ϵ�д�
����ȫ���ִʾ��ƪ��ϵ�д� �����߿����ϵ�д�
�����߿����ϵ�д�
| �꼶 | ���пγ� | �꼶 | ���пγ� |
| ��һ | ��һ��ѿγ��Ƽ��� | ��һ | ��һ��ѿγ��Ƽ��� |
| �߶� | �߶���ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
| ���� | ������ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
��Ŀ�����л�ѧ ��Դ����ȫ��У���������Ĵ�ʡ���ؾ�У2017����꼶��ѧ�ڵڶ�������ģ�⻯ѧ�Ծ� ���ͣ�ѡ�������
ѧУ���Ļ���Ҷɫ���ơ��������ݴˣ�����ʩ�õĻ����ǣ� ��
A. KH2PO4 B. KNO3 C. CO(NH2)2 D. Ca3(PO4)2
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ������ʡ�ܿ����е�����ģ�⿼�Ի�ѧ�Ծ� ���ͣ�ѡ�������
һ�������£��������ʵ�ת������һ��ʵ�ֵ���
A. AgNO3�� Fe(NO3)2�� Zn(NO3)2 B. CaCl2�� CaCO3�� Ca(OH)2
C. NaNO3�� NaOH�� Na2CO3 D. Fe2O3�� FeSO4��Fe(OH)3
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ����ȫ���б�ҵ�����ģ�⿼�����ۻ�ѧ�Ծ� ���ͣ���Ϣ������
��ͼ��ʾ��a��b��c���ֹ������ʵ��ܽ�����ߡ������ͼʾ��գ�

��1�� �����¶ȵ��������ܽ�ȷ�����С����_____����___��ʱ��a��b���ܽ����ȡ�
��2�� t3��ʱ��a���ʵı�����Һ�У����ʡ��ܼ�����Һ������֮��Ϊ___________��
��3�� �ɸ��ܽ�����߿�֪����a�ı�����Һ����ȡa���ʣ�Ӧ���õķ�����_____________��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ����ȫ���б�ҵ�����ģ�⿼�����ۻ�ѧ�Ծ� ���ͣ�ѡ�������
����ʳ���У��������۵��ǣ� ��

A. A B. B C. C D. D
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ������ʡ������2017����꼶��ѧ�ڵ�һ��ģ�⿼�Ի�ѧ�Ծ� ���ͣ�̽����
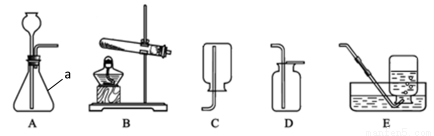
���������ǻ������õ�����Դ����������װ�ûش�
��1����Aװ�õ��ˮʵ�飬�ڼ��Թ����ռ�����������_________��
��2��ʵ������ȡ��������������ҩƷ�е�________������ţ�
������ϡ���� ��п��ϡ���� ������п��ϡ����
��3��Bװ��������ȼ�ϵ�أ�����������������Ӧ��________��ֱ��ת��Ϊ���ܡ���������������ɴ���̫��վ�Ⱥ�������ʹ�ã������IJ���Ϊ_______�����ɹ��Ա���ã�
��4���о���Ա�����з���CO2�������̴ѡ���ȼ�ϵļ�������CO2��H2��Ϻ�ͨ�������������ӵ�ϸ���ܵ������ȣ��ڹܵ���һ�˳����ľ���ȼ�ϡ���Ӧԭ��Ϊ�� ������X�Ļ�ѧʽΪ_________�������������ڷ�Ӧ����_________���á�
������X�Ļ�ѧʽΪ_________�������������ڷ�Ӧ����_________���á�
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ������ʡ������2017����꼶��ѧ�ڵ�һ��ģ�⿼�Ի�ѧ�Ծ� ���ͣ�ѡ�������
����Ϊ�˴ﵽʵ��Ŀ�Ķ����е�ʵ�������������ȷ���ǣ� ��
ѡ�� | ʵ��Ŀ�� | ʵ����� |
A | ϴ���ڱ�մ����֬���Թ� | �ô�����ˮ��ϴ |
B | ���鼯��ƿ��O2�Ƿ��� | ��ȼ�ŵ�ľ�����뼯��ƿ |
C | ���CO���Ƿ����CO2 | ������ͨ�����ʯ��ˮ |
D | ��ȥKCl��ĩ�л��е�KClO3 | ��MnO2������ |
A. A B. B C. C D. D
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ����ȫ����ǿУ������ʡ������2017����꼶��ѧ�ڵ�һ���¿���ѧ�Ծ� ���ͣ�̽����
��ѧ��һ����ʵ��Ϊ�����Ŀ�ѧ������ͼʾ�ش����⣺
��1�������a������_________________��
��2��ʵ�����ø��������ȡ��������Ӧ�Ļ�ѧ����ʽΪ___________________________________________����Ӧѡ��ķ���װ����_______������ĸ�����ռ������Ͷ�����̼������ѡ���װ����_______������ĸ����
��3����ʵ������Aװ����ȡCO2��Ϊ�˵õ����������CO2���壬����ͬѧ���������ͼ����װ�á���F����Һ��������______________________��G�е�Һ����_______________������Hװ���ռ�CO2������Ӧ��________���a����b����ͨ�룩��

�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ������ʡ������2017����꼶��ѧ�ڵڶ���ģ�⿼�Ի�ѧ�Ծ� ���ͣ�ѡ�������
�����Դ��������ˮ��Դ�ȷ����Σ��������4�������к������е���
A. �������ɵ���ˮ������������ˮ������
B. �������Ͻ�ʹ��������Ʒ���Խ������ɫ��Ⱦ������
C. ��Խ������ﴢ������Σ����Ѱ�ҽ��������Ʒ
D. ��ֹ������ʹ�û�ʯȼ�ϣ���Ӧ��ȫ����ԴΣ��
�鿴�𰸺ͽ���>>
����ʡ������Υ���Ͳ�����Ϣ�ٱ�ƽ̨ | �����к���Ϣ�ٱ�ר�� | ����թƭ�ٱ�ר�� | ����ʷ���������к���Ϣ�ٱ�ר�� | ������Ȩ�ٱ�ר��
Υ���Ͳ�����Ϣ�ٱ��绰��027-86699610 �ٱ����䣺58377363@163.com