
 CO��+CO2��+H2O��
CO��+CO2��+H2O�� CO��+CO2��+H2O��
CO��+CO2��+H2O��


| �꼶 | ���пγ� | �꼶 | ���пγ� |
| ��һ | ��һ��ѿγ��Ƽ��� | ��һ | ��һ��ѿγ��Ƽ��� |
| �߶� | �߶���ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
| ���� | ������ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
 ��ʾ
��ʾ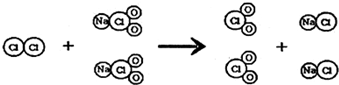
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ��Ķ�����
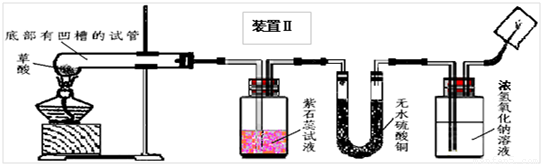
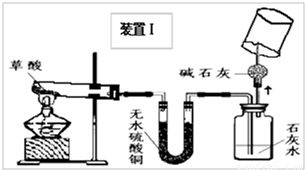
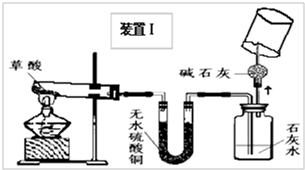 ��2008?��ɽ��һģ��ij��ѧ��ѧѧϰС�����Ķ��ˡ���ȥ֯�������յķ��������ֲ����ܹ����ڳ�ȥˮ���ա�Ѫ�ա����⡢��īˮ�����գ��Բ��������Ũ�����Ȥ����ʦ����ͬѧ�Dz���Ļ�ѧʽΪH2C2O4�����ȷֽ⣮ͬѧ�ǶԲ������ȷֽ����̽����
��2008?��ɽ��һģ��ij��ѧ��ѧѧϰС�����Ķ��ˡ���ȥ֯�������յķ��������ֲ����ܹ����ڳ�ȥˮ���ա�Ѫ�ա����⡢��īˮ�����գ��Բ��������Ũ�����Ȥ����ʦ����ͬѧ�Dz���Ļ�ѧʽΪH2C2O4�����ȷֽ⣮ͬѧ�ǶԲ������ȷֽ����̽����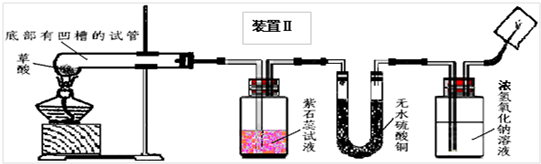
| ||
| ||
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ����ѧ������ ���ͣ�058
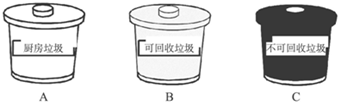
ij��ѧ̽��ѧϰС���ڰ���ʵ����������ѧ�Լ�ʱ������һʢ����ɫ��Һ
(���꼶��ѧ�����Լ���ij���ơ�)���Լ�ƿ����ǩ����(��ͼ)����Ը��Լ��������룬�����ʵ����֤��
(1)
���룺�����Լ�������________��Һ��Ҳ������________��Һ��(2)
ʵ����֤(Ҫ��д��������������)��________________��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ����ѧ������ ���ͣ�059
ij��ѧ̽��ѧϰС���ڰ���ʵ����������ѧ�Լ�ʱ������һ��ʢ����ɫ��Һ
(���л�ѧ�����Լ�)���Լ�ƿ����ǩ����(��ͼ)������Ը��Լ��������룬�����ʵ����֤��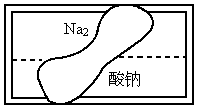
�鿴�𰸺ͽ���>>
����ʡ������Υ���Ͳ�����Ϣ�ٱ�ƽ̨ | �����к���Ϣ�ٱ�ר�� | ����թƭ�ٱ�ר�� | ����ʷ���������к���Ϣ�ٱ�ר�� | ������Ȩ�ٱ�ר��
Υ���Ͳ�����Ϣ�ٱ��绰��027-86699610 �ٱ����䣺58377363@163.com