��2013?ƽ������ģ����д��������صĻ�ѧ��Ӧ����ʽ����������գ�
��1����˿�ڴ���������ȼ��
�����и�ʵ��ʱ������ƿ�ײ����е�ˮ��������
��ֹ���½�����ը��ƿ��
��ֹ���½�����ը��ƿ��
��
��2����ҵ����һ����̼��ԭ������ұ�������ķ�Ӧ��
�豸��������
��¯
��¯
��
��3����������β������������������Һ���գ�
SO2+2NaOH�TNa2SO3+H2O
SO2+2NaOH�TNa2SO3+H2O
��4����Ϊ�����Ȼ�茶��壨NH
4Cl������ʯ�һ����ĥ������һ�ְ���ζ�����壬�Ӷ����ͷ�Ч
2NH4Cl+Ca��OH��2�TCaCl2+2H2O+2NH3��
2NH4Cl+Ca��OH��2�TCaCl2+2H2O+2NH3��
��������������ʪ���
��ɫʯ��
��ɫʯ��
��ֽ��������
��ɫʯ����ֽ����ɫ
��ɫʯ����ֽ����ɫ
��
��5�����������Ũ����մ��Ƥ�����·��ϣ�Ӧ�����ô�����ˮ��ϴ��Ȼ����Ϳ��3%��5%��NaHC0
3��Һ
2NaHCO3+H2SO4=Na2SO4+2H2O+2CO2��
2NaHCO3+H2SO4=Na2SO4+2H2O+2CO2��
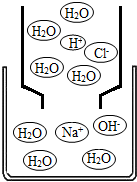
��6����ͼ��ʾ�ķ�Ӧ��
HCl+NaOH=NaCl+H2O
HCl+NaOH=NaCl+H2O
���÷�Ӧ�Ļ���������
���ֽⷴӦ
���ֽⷴӦ
������
��
��
�ȷ�Ӧ��������š�����

 ��2013?ƽ������ģ����д��������صĻ�ѧ��Ӧ����ʽ����������գ�
��2013?ƽ������ģ����д��������صĻ�ѧ��Ӧ����ʽ����������գ�

