
 ijУ�о���ѧϰС�������һ����Ȥ��ʵ��̽����
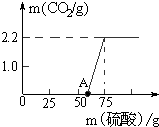
ijУ�о���ѧϰС�������һ����Ȥ��ʵ��̽�������� ̼���ƺ�ϡ���ᷴӦ���������ơ�ˮ�Ͷ�����̼�����ݶ�����̼���������Լ���̼���Ƶ���������һ�����Լ����������Ƶ���������Ʒ��NaOH������������
��� �⣺��1���⣺�����Ʒ��Na2CO3������Ϊx������H2SO4������Ϊy����ͼ�����ݿ�֪�����ɶ�����̼������Ϊ2.2g������ϡ���������Ϊ75g-50g=25g
Na2CO3+H2SO4�TNa2SO4+H2O+CO2��
106 98 44
x y 2.2g
$\frac{106}{x}=\frac{44}{2.2g}$
x=5.3g
$\frac{98}{y}=\frac{44}{2.2g}$
y=4.9g
����Ʒ��NaOH����������Ϊ$\frac{13.3g-5.3g}{13.3g}��$100%=60.2%��
��ϡ�������������Ϊ��$\frac{4.9g}{25g}��$100%=19.6%
�𰸣�
��1������Ʒ��Na2CO3������Ϊ5.3g��
��2������Ʒ��NaOH����������Ϊ60.2%��
��3����ϡ�������������Ϊ19.6%��
���� ������Ҫ����ѧ�����ü��跨�ͻ�ѧ����ʽ���м�����ƶϵ�����������ʱҪע��淶�Ժ�ȷ�ԣ�


 ��У����ϵ�д�
��У����ϵ�д�
| �꼶 | ���пγ� | �꼶 | ���пγ� |
| ��һ | ��һ��ѿγ��Ƽ��� | ��һ | ��һ��ѿγ��Ƽ��� |
| �߶� | �߶���ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
| ���� | ������ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
��Ŀ�����л�ѧ ��Դ�� ���ͣ������
 �ҹ��̲��ŷḻ�ĺ�����Դ������ˮ��Դ��ȱ����Ҫ�������ã�����ˮ��Դ��
�ҹ��̲��ŷḻ�ĺ�����Դ������ˮ��Դ��ȱ����Ҫ�������ã�����ˮ��Դ���鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ������
 2010��3��14�գ�ij�о����˳��п�ѧ֪ʶӦ����̽�����ܱ��������е�Ҷ����ǩ������Ŀ����ѡ���ڹ涨ʱ���ڣ��������ѵĴ�����ƣ���Ҷ������Ⱦɫ���滭���øƴ�ӵ������ӹ�������ʱij��ѡ��������200�� 10%������������Һ���������������Ƕ�ʣ����������Ʒ�Һ������������кʹ�����
2010��3��14�գ�ij�о����˳��п�ѧ֪ʶӦ����̽�����ܱ��������е�Ҷ����ǩ������Ŀ����ѡ���ڹ涨ʱ���ڣ��������ѵĴ�����ƣ���Ҷ������Ⱦɫ���滭���øƴ�ӵ������ӹ�������ʱij��ѡ��������200�� 10%������������Һ���������������Ƕ�ʣ����������Ʒ�Һ������������кʹ������鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�ѡ����
| A�� | ʯ�� | B�� | ľ�� | C�� | ��˿ | D�� | �ƾ� |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ������
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ������
| ���� | �о������ɫ��ζ | ����ͨ����ɫʯ����Һ | ����ͨ�����ʯ��ˮ |
| ���� | ��ɫ����ζ | ��ɫʯ����Һ���ɫ | ����ʯ��ˮ����� |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�ѡ����
| A�� | a��b | B�� | a��b | C�� | a=b | D�� | ��ϵ |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ������
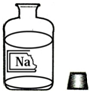 ��ѧ��ȤС���ͬѧ��ʵ���ҷ���һƿ���ܲ�����ʢ����ɫ��Һ���Լ�ƿ���ұ�ǩ������ͼ�������ȱ�ı�ǩ��ֻʣ�¡�Na����������ʦ�������ǣ���ƿ��Һ�е����ʿ������������ơ��Ȼ��ƻ�̼���ƣ�ͬѧ�Ǻܸ���Ȥ����������ɷֽ���̽����
��ѧ��ȤС���ͬѧ��ʵ���ҷ���һƿ���ܲ�����ʢ����ɫ��Һ���Լ�ƿ���ұ�ǩ������ͼ�������ȱ�ı�ǩ��ֻʣ�¡�Na����������ʦ�������ǣ���ƿ��Һ�е����ʿ������������ơ��Ȼ��ƻ�̼���ƣ�ͬѧ�Ǻܸ���Ȥ����������ɷֽ���̽����| ʵ�� | ʵ����� | ��Ҫʵ������ | ʵ����ۺͽ��� |
| 1 | ȡ�����Թ��У������еμ�2����ɫ��̪��Һ�� | ��ɫ��Һ���ɫ | ����Һ��������NaCl��Һ ������NaCl��Һ�����ԣ�����ʹ��̪��ɫ |
| 2 | ȡ������һ֧�Թ��У������еμ�����ϡ���ᣮ | �������������� | ����Һ������Ϊ̼���� |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ������
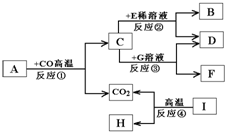 A��I��ʾ���ֳ��л�ѧ�г����Ĵ������֪A�dz��������Ҫ�ɷ֣�E��Ũ��Һϡ��ʱ��ų��������ȣ�F�Ǻ�ɫ���嵥�ʣ�I�������������ϣ�����֮�������ͼ��ʾ��ת����ϵ��
A��I��ʾ���ֳ��л�ѧ�г����Ĵ������֪A�dz��������Ҫ�ɷ֣�E��Ũ��Һϡ��ʱ��ų��������ȣ�F�Ǻ�ɫ���嵥�ʣ�I�������������ϣ�����֮�������ͼ��ʾ��ת����ϵ���鿴�𰸺ͽ���>>
����ʡ������Υ���Ͳ�����Ϣ�ٱ�ƽ̨ | �����к���Ϣ�ٱ�ר�� | ����թƭ�ٱ�ר�� | ����ʷ���������к���Ϣ�ٱ�ר�� | ������Ȩ�ٱ�ר��
Υ���Ͳ�����Ϣ�ٱ��绰��027-86699610 �ٱ����䣺58377363@163.com