
(10��)���ͼʾʵ��װ�ã��ش��������⡣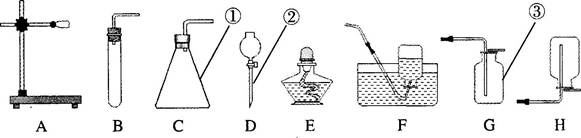
(1)д�����������ƣ���_______����_______����_______��
(2)�����ռ����巽���������ǣ�F_______��G_______����
(3)ʵ����ʹ�ø�������Ʊ�������ѡ�õ�װ�����Ϊ_______��
(4)��˫��ˮ�Ͷ��������Ʊ���������Ӧ�Ļ�ѧ����ʽΪ____________________________��
����Fװ���ռ������� ��______ʱ�� ֤��������������Ӧ��Һ�еĶ������̲�Ҫ�����������ʹ�ã��ɲ���_______��ϴ�Ӻ���Ȳ������õ�����������̡�

| �꼶 | ���пγ� | �꼶 | ���пγ� |
| ��һ | ��һ��ѿγ��Ƽ��� | ��һ | ��һ��ѿγ��Ƽ��� |
| �߶� | �߶���ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
| ���� | ������ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
(10��)���ͼʾʵ��װ�ã��ش��������⡣
(1)д�����������ƣ���_______����_______����_______��
(2)�����ռ����巽���������ǣ�F_______��G_______����
(3)ʵ����ʹ�ø�������Ʊ�������ѡ�õ�װ�����Ϊ_______��
(4)��˫��ˮ�Ͷ��������Ʊ���������Ӧ�Ļ�ѧ����ʽΪ____________________________��
����Fװ���ռ������� ��______ʱ�� ֤��������������Ӧ��Һ�еĶ������̲�Ҫ�����������ʹ�ã��ɲ���_______��ϴ�Ӻ���Ȳ������õ�����������̡�
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�

�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ��2012����б�ҵ��ѧ���ԣ��������ݾ�����ѧ���������� ���ͣ��ʴ���
(10��)�������ͼʾʵ��װ�ã��ش��й����⣺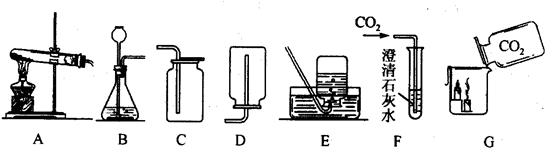
(1)ʵ�����ø��������ȡ����ʱ��Ӧѡ�õķ���װ���� (����ĸ��ͬ)����Ӧ�Ļ�ѧ����ʽΪ ���Թܿڷ�һ������������ ��
(2)ʵ������ȡ������̼ʱ��Ӧѡ��ķ������ռ�װ���� ��
(3)F�й۲쵽��ʵ�������� ��
��Ӧ�Ļ�ѧ����ʽΪ ��
(4)Gʵ����˵��������̼���е������� ����CO2������ ��
(5)�����£�����(H2S)��һ���г�������ζ�����壺ʵ���ҿ���������(FeS)�����ϡ���ᷴӦ�Ƶã��÷�Ӧ�Ļ�ѧ����ʽΪFeS+H2S04=H2S��+FeSO4.ʵ������ȡ��������Ӧѡ�õķ���װ���� �������� ��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ��2012�콭��ʡ�����о��꼶��һѧ����ĩ���Ի�ѧ�Ծ� ���ͣ������
(10��)���ͼʾʵ��װ�ã��ش��������⡣

(1)д�����������ƣ���_______����_______����_______��
(2)�����ռ����巽���������ǣ�F_______��G_______����
(3)ʵ����ʹ�ø�������Ʊ�������ѡ�õ�װ�����Ϊ_______��
(4)��˫��ˮ�Ͷ��������Ʊ���������Ӧ�Ļ�ѧ����ʽΪ____________________________��
����Fװ���ռ������� ��______ʱ�� ֤��������������Ӧ��Һ�еĶ������̲�Ҫ�����������ʹ�ã��ɲ���_______��ϴ�Ӻ���Ȳ������õ�����������̡�
�鿴�𰸺ͽ���>>
����ʡ������Υ���Ͳ�����Ϣ�ٱ�ƽ̨ | �����к���Ϣ�ٱ�ר�� | ����թƭ�ٱ�ר�� | ����ʷ���������к���Ϣ�ٱ�ר�� | ������Ȩ�ٱ�ר��
Υ���Ͳ�����Ϣ�ٱ��绰��027-86699610 �ٱ����䣺58377363@163.com