�⣺��1������������ȼ���������������������Ժ�ɫ������������������
��2����Ӧ��ķ����к���2����ԭ�ӡ�һ����ԭ�ӡ�2����ԭ�ӣ����������Ѿ�����һ����ԭ�ӣ���ȱ��2����ԭ�Ӻ�2����ԭ�ӣ����Բ����������2HCl�������ڷ�Ӧǰ��û�䣬���ڴ�����������������ýд����ã�
��3������������ˮ���Ȼ���������ˮ�����Լ�ˮ�ܽ⣬�ٽ��й��ˡ�ϴ�Ӹ��T�ɣ�
��������0.7g������Ҫ�μӷ�Ӧ����������ΪX����
Fe+2HCl�TFeCl
2+H
2��
56 2
X 0.7g
���ݣ�
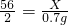
���X=19.6g��
�ʴ�Ϊ����1��Fe
3O
4����2��2HCl�������ã���3����ˮ�����ˣ���������0.7g������Ҫ�μӷ�Ӧ����������ΪX����
Fe+2HCl�TFeCl
2+H
2��
56 2
X 0.7g
���ݣ�
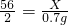
���X=19.6g��
��������1����������������Ӧ�����������������ǣ���2�����ݷ�Ӧǰ�����ԭ���������Ŀ����ʹ������ص㿼�ǣ���3���ٸ�����������ˮ���Ȼ���������ˮ���л����ķ��룻�ڸ�����������������������������ٳ���20g�������������������
�����������ؼ���֪����ȼ�յIJ������������������ܹ�������������غ㶨�ɽ��ʵ�����⣬֪�������Թ����Һ�����ķ�������Ϥ���ݷ���ʽ�ļ��㷽����


 Fe+______��
Fe+______��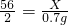 ���X=19.6g��
���X=19.6g��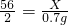 ���X=19.6g��
���X=19.6g��