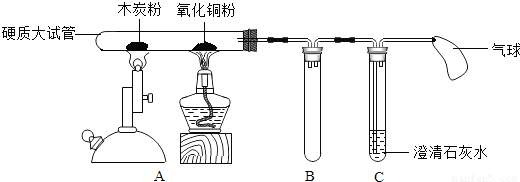
ij��ʦ��������ʾ��װ�ý���ʵ�飨ͼ�й̶��Թܵ���������ȥ��������һ��ʱ��۲쵽ľ̿�۵��������Լ��٣�����һ������ʣ�ࣻ����ͭ���²�������һ������ɫ��ͭ������ʯ��ˮ����ǣ�

��1��ľ̿��������������Ϊ�ڸ���������ľ̿������Թ���������Ӧ��������Ӧ�ķ���ʽΪ
��
��2������ͭ��ĩ�²�������һ������ɫ��ͭ��������Ӧ�ķ���ʽΪ
��
��3��ʯ��ˮ����ǵķ���ʽ
CO2+Ca��OH��2�TCaCO3��+H2O
CO2+Ca��OH��2�TCaCO3��+H2O
��4��ʵ�������Ϩ��Ӳ�ʴ��Թ��µľƾ��ƺ;ƾ���ƣ�ֱ�����Թ���ȴ���ٳ���������C�е�Һ�����ŵ�������Bװ���У�������������ԭ��
ֹͣ���Ȳ���ȴ������ʱ���Թ��е�ѹǿ��С���ڴ���ѹ�������£�װ��C�е�Һ�嵹����װ��C��
ֹͣ���Ȳ���ȴ������ʱ���Թ��е�ѹǿ��С���ڴ���ѹ�������£�װ��C�е�Һ�嵹����װ��C��
��





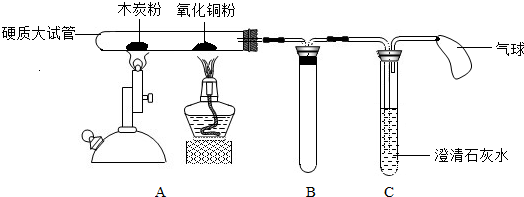
 ij��ʦ��������ʾ��װ�ý���ʵ�飨ͼ�й̶��Թܵ���������ȥ��������һ��ʱ��۲쵽ľ̿�۵��������Լ��٣�����һ������ʣ�ࣻ����ͭ���²�������һ������ɫ��ͭ������ʯ��ˮ����ǣ�
ij��ʦ��������ʾ��װ�ý���ʵ�飨ͼ�й̶��Թܵ���������ȥ��������һ��ʱ��۲쵽ľ̿�۵��������Լ��٣�����һ������ʣ�ࣻ����ͭ���²�������һ������ɫ��ͭ������ʯ��ˮ����ǣ�