
(7��)ˮ����Ҫ����Դ������Ӧ�˽�ˮ���й�֪ʶ��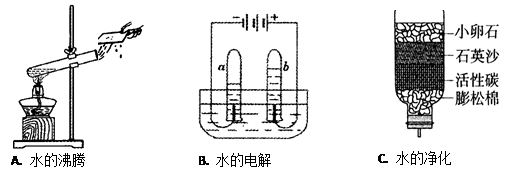
(1)A��ˮ������ �����������ѧ�����仯��
(2)B�ǵ��ˮ��ʵ��װ�ã�д���÷�Ӧ�����ֱ���ʽ�� ����
����b�Թ��в��������塣
(3) ��ͬѧ������ѧ��֪ʶ�����ǵĿ�ˮ��Cͼ��ʾ�ļ���ˮ�����о���������С��ʯ��ʯӢɳ�������� ��
(4)Ӳˮ����������������ܶ��鷳�������п��� ������Ӳˮ����ˮ������ �ķ���������ˮ��Ӳ�ȡ�
��5�������ϵ��ܴ�ˮ����Ȼ�ܴ���ˮ���٣�����ˮ��Դ��ÿ����������κ�����������Ϊ���ڽ�Լ��ˮ���� (�����)
A.���������������ֹ�ˮ��ͷ B��ϴ��˵�ˮ�������� C.����Ϸ�ˮˢ��
��1������ ˮ ������+������2�������ǵ�ľ����3�����ˣ�4������ˮ ���
��5��BC
���������������1��ˮ�ķ��ڵĹ����У�ˮ��Һ̬�����̬�����Թܿ��ֱ��Һ̬������ˮ��״̬�ı仯��û�в����µ����ʣ��������������仯��
��2�����ˮ��ʵ���У������õ������������������õ�������������b���е�������������������������֧��ȼ�յ����ʣ�����ѡ�ô����ǵ�ľ�������飻
��3������ˮ�ľ������̼���������֪��С��ʯ��ʯӢɳ������Ϊ���˳�������ˮ�����ʣ�
��4������Ӳˮ����ˮ���÷���ˮ�������ˮ���ã���������Ӳˮ������ĭ�������ˮ ��
��5��A�����������������ֹ�ˮ��ͷ�������˷�ˮ������������ȡ��
B��ϴ��˵�ˮ�����������������������ˮ�������ʣ��ɽ�Լ��ˮ��
C������Ϸ�ˮˢ��������˷�ˮ����ѡB��
���㣺ˮ�ľ��������ˮʵ�飬Ӳˮ����ˮ������ˮ��Դ�ͽ�Լ��ˮ
�����������Ĺؼ���Ҫ���վ���ˮ�ͺ͵��ˮ�����֪ʶ��ֻ���������ܶ�����������ȷ���жϣ����Ҫ֪������Ӳˮ����ˮ�÷���ˮ�������ɽ�Լ��ˮ��ϰ�ߡ�



| �꼶 | ���пγ� | �꼶 | ���пγ� |
| ��һ | ��һ��ѿγ��Ƽ��� | ��һ | ��һ��ѿγ��Ƽ��� |
| �߶� | �߶���ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
| ���� | ������ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
��Ŀ�����л�ѧ ��Դ�� ���ͣ�

�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ��2012-2013ѧ���Ĵ�ʡ���꼶��ѧ�����п��Ի�ѧ�Ծ��������棩 ���ͣ������
(7��)ˮ����Ҫ����Դ������Ӧ�˽�ˮ���й�֪ʶ��
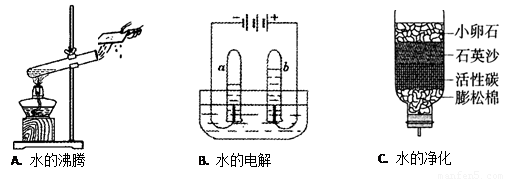
(1)A��ˮ������ �����������ѧ�����仯��
(2)B�ǵ��ˮ��ʵ��װ�ã�д���÷�Ӧ�����ֱ���ʽ�� ����
����b�Թ��в��������塣
(3) ��ͬѧ������ѧ��֪ʶ�����ǵĿ�ˮ��Cͼ��ʾ�ļ���ˮ�����о���������С��ʯ��ʯӢɳ�������� ��
(4)Ӳˮ����������������ܶ��鷳�������п��� ������Ӳˮ����ˮ������ �ķ���������ˮ��Ӳ�ȡ�
��5�������ϵ��ܴ�ˮ����Ȼ�ܴ���ˮ���٣�����ˮ��Դ��ÿ����������κ�����������Ϊ���ڽ�Լ��ˮ���� (�����)
A.���������������ֹ�ˮ��ͷ B��ϴ��˵�ˮ�������� C.����Ϸ�ˮˢ��
�鿴�𰸺ͽ���>>
����ʡ������Υ���Ͳ�����Ϣ�ٱ�ƽ̨ | �����к���Ϣ�ٱ�ר�� | ����թƭ�ٱ�ר�� | ����ʷ���������к���Ϣ�ٱ�ר�� | ������Ȩ�ٱ�ר��
Υ���Ͳ�����Ϣ�ٱ��绰��027-86699610 �ٱ����䣺58377363@163.com