
��2011���㽭���ݣ�38�⣩ijѧУ��ѧ��ȤС�������һ��ʵ�飬��ģ���о�CO2 ��Ũ�������Ƿ������������ЧӦ�������Dz������й����ݣ�

������������ʵ��Ͳ������裺
������ֻͬ���IJ���ƿ��ֱ����CO2�Ϳ����������Ϊ�ס��ң���������ͬ���¶ȼƵ���Ƥ�����ٰ���ֻ����ƿ�������������䣨����ͼ�����۲�ס���ƿ�е��¶ȱ仯��

��.����������䣬���һ��ʱ�����������ƿ�¶�ֵ������¼�����±���

��ش��������⣺
(1)��д��ʵ������ȡCO2�Ļ�ѧ����ʽ ��
(2)����ƿ�г�CO2ʱ����֤ƿ���ѳ�����CO2�ķ����� ��
(3)����ʵ���У�����ͬ��ʱ�䣬�����ϱ������ݣ��Ƚϼס���ƿ�¶ȱ仯�Ĺ�����
��
(4)����ʵ���У������������£�Ӱ��ס���ƿ�¶Ȳ�ͬ��ԭ����CO2������ЧӦ���⣬���еĿ���ԭ���ǣ�д��һ�㼴�ɣ�
(5)��ͬѧ��Ϊ���ݸ�ģ��ʵ���Ŀ�ģ�ʵ����ƴ������⣬����Ϊ�ǣ�д��һ�㼴�ɣ�
��
 ȫ�ų��100��ϵ�д�
ȫ�ų��100��ϵ�д� Ӣ�ŵ��ϵ�д�
Ӣ�ŵ��ϵ�д�
| �꼶 | ���пγ� | �꼶 | ���пγ� |
| ��һ | ��һ��ѿγ��Ƽ��� | ��һ | ��һ��ѿγ��Ƽ��� |
| �߶� | �߶���ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
| ���� | ������ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
��2011���㽭���ݣ� 30�⣩Ϊ�˼��������Σ�����ҹ��з��ˡ���ˮ�����������ա����乤�����̴����ǣ�
I���Ӹߴ������µĺ�ˮ(pH=8.1-8.3)ϴ�Ѵ��¶���ȼú�����е�SO2, (SO2+H2O=H2SO3 H2SO3�����ᣩ����ˮ��Ϊ�����ԣ�
��.��ϴ��������ĺ�ˮ��������ֽӴ����������з�Ӧ��
4NaC1��O2ʮ2H2SO3 ==2Na2SO4��4HC1
III.�ٽ���������ֽӴ��ĺ�ˮ��������Ȼ��ˮ��ϵȴ�ʩ��ʹ����ָ��ӽ���Ȼ��ˮ�����ŷš�
(1)��������ͼ�л�������ˮ�����������ա�ȫ�����к�ˮ��pH���α仯�������ơ�
(2)��������������Ȼ��ˮ�Ӵ��������Ϊ�����ԣ���˵���ڴ˹����к�ˮ�е� ���Ӻ�H2SO3������������ӷ����˷�Ӧ��
(3)����Ҫ�ⶨ��ˮ��Na2SO4������ʵ����������ǣ�
ȡ�������⺣ˮ���ⶨ ������������ ��Һ��д��ѧʽ�����ټ���ϡ���ᡢ���ˡ�ϴ�ӡ���ɡ���������������
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ��2011��ȫ���п���ѧ����������13 ������̼��ȡ������ ���ͣ��ʴ���
��2011���㽭���ݣ�38�⣩ijѧУ��ѧ��ȤС�������һ��ʵ�飬��ģ���о�CO2Ũ�������Ƿ������������ЧӦ�������Dz������й����ݣ�������������ʵ��Ͳ������裺
������ֻͬ���IJ���ƿ��ֱ����CO2�Ϳ����������Ϊ�ס��ң���������ͬ���¶ȼƵ���Ƥ�����ٰ���ֻ����ƿ�������������䣨����ͼ�����۲�ס���ƿ�е��¶ȱ仯��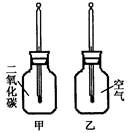
������������䣬���һ��ʱ�����������ƿ�¶�ֵ������¼�����±���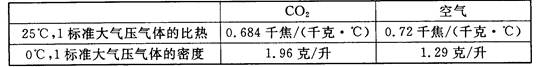
��ش��������⣺
(1)��д��ʵ������ȡCO2�Ļ�ѧ����ʽ ��
(2)����ƿ�г�CO2ʱ����֤ƿ���ѳ�����CO2�ķ����� ��
(3)����ʵ���У�����ͬ��ʱ�䣬�����ϱ������ݣ��Ƚϼס���ƿ�¶ȱ仯�Ĺ�����
��
(4)����ʵ���У������������£�Ӱ��ס���ƿ�¶Ȳ�ͬ��ԭ����CO2������ЧӦ���⣬���еĿ���ԭ���ǣ�д��һ�㼴�ɣ�
ͬѧ��Ϊ���ݸ�ģ��ʵ���Ŀ�ģ�ʵ����ƴ������⣬����Ϊ�ǣ�д��һ�㼴�ɣ�
��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ��2011��ȫ���п�����ר����ר���� ̽���⣨һ�� ���ͣ�̽����
��2011���㽭���ݣ� 30�⣩Ϊ�˼��������Σ�����ҹ��з��ˡ���ˮ�����������ա����乤�����̴����ǣ�
I���Ӹߴ������µĺ�ˮ(pH=8.1-8.3)ϴ�Ѵ��¶���ȼú�����е�SO2, (SO2+H2O=H2SO3 H2SO3�����ᣩ����ˮ��Ϊ�����ԣ�
��.��ϴ��������ĺ�ˮ��������ֽӴ����������з�Ӧ��
4NaC1��O2ʮ2H2SO3 ==2Na2SO4��4HC1
III.�ٽ���������ֽӴ��ĺ�ˮ��������Ȼ��ˮ��ϵȴ�ʩ��ʹ����ָ��ӽ���Ȼ��ˮ�����ŷš�
(1)��������ͼ�л�������ˮ�����������ա�ȫ�����к�ˮ��pH���α仯�������ơ�
(2)��������������Ȼ��ˮ�Ӵ��������Ϊ�����ԣ���˵���ڴ˹����к�ˮ�е� ���Ӻ�H2SO3������������ӷ����˷�Ӧ��
(3)����Ҫ�ⶨ��ˮ��Na2SO4������ʵ����������ǣ�
ȡ�������⺣ˮ���ⶨ ������������ ��Һ��д��ѧʽ�����ټ���ϡ���ᡢ���ˡ�ϴ�ӡ���ɡ���������������
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ��2011��ȫ���п�����ר����ר���� ̽���⣨һ�� ���ͣ�̽����
��2011���㽭���ݣ�38�⣩ijѧУ��ѧ��ȤС�������һ��ʵ�飬��ģ���о�CO2��Ũ�������Ƿ������������ЧӦ�������Dz������й����ݣ�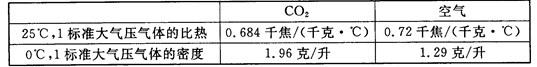
������������ʵ��Ͳ������裺
������ֻͬ���IJ���ƿ��ֱ����CO2�Ϳ����������Ϊ�ס��ң���������ͬ���¶ȼƵ���Ƥ�����ٰ���ֻ����ƿ�������������䣨����ͼ�����۲�ס���ƿ�е��¶ȱ仯��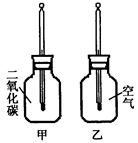
��.����������䣬���һ��ʱ�����������ƿ�¶�ֵ������¼�����±���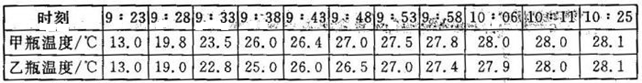
��ش��������⣺
(1)��д��ʵ������ȡCO2�Ļ�ѧ����ʽ ��
(2)����ƿ�г�CO2ʱ����֤ƿ���ѳ�����CO2�ķ����� ��
(3)����ʵ���У�����ͬ��ʱ�䣬�����ϱ������ݣ��Ƚϼס���ƿ�¶ȱ仯�Ĺ�����
��
(4)����ʵ���У������������£�Ӱ��ס���ƿ�¶Ȳ�ͬ��ԭ����CO2������ЧӦ���⣬���еĿ���ԭ���ǣ�д��һ�㼴�ɣ�
��ͬѧ��Ϊ���ݸ�ģ��ʵ���Ŀ�ģ�ʵ����ƴ������⣬����Ϊ�ǣ�д��һ�㼴�ɣ�
��
�鿴�𰸺ͽ���>>
����ʡ������Υ���Ͳ�����Ϣ�ٱ�ƽ̨ | �����к���Ϣ�ٱ�ר�� | ����թƭ�ٱ�ר�� | ����ʷ���������к���Ϣ�ٱ�ר�� | ������Ȩ�ٱ�ר��
Υ���Ͳ�����Ϣ�ٱ��绰��027-86699610 �ٱ����䣺58377363@163.com