
| ||
| ||
| 117 |
| y |
| 2 |
| x |
| 71 |
| 7.1g |
| 36g |
| 100g+36g |
| 14.8g |
| 100g-0.2g-7.1g |


 ���100��1�ž�ϵ�д�
���100��1�ž�ϵ�д�
| �꼶 | ���пγ� | �꼶 | ���пγ� |
| ��һ | ��һ��ѿγ��Ƽ��� | ��һ | ��һ��ѿγ��Ƽ��� |
| �߶� | �߶���ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
| ���� | ������ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
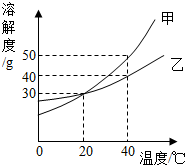 ��ͼ�Ǽס������ֹ������ʵ��ܽ�����ߣ�����˵����ȷ���ǣ�������
��ͼ�Ǽס������ֹ������ʵ��ܽ�����ߣ�����˵����ȷ���ǣ�������| A��20��ʱ���ס�����Һ�����ʵ������������ |
| B��20��ʱ����60g����100gˮ�еõ���ҺA�����ټ���100gˮ����ʹ��ҺA������������С |
| C��40��ʱ���ı�����Һ�����ʵ���������ԼΪ33.3% |
| D���ɲ��ý����¶ȵķ�����ʹ�ҵı�����Һ��Ϊ��������Һ |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
 ��ͼ��ʾ��A��H�dz��л�ѧ���������ʣ�A��B�ɷ����кͷ�Ӧ��X������ʳƷ�������YΪ���ʣ�����ͼʾ�ش��������⣺
��ͼ��ʾ��A��H�dz��л�ѧ���������ʣ�A��B�ɷ����кͷ�Ӧ��X������ʳƷ�������YΪ���ʣ�����ͼʾ�ش��������⣺�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
| ʵ����� | ʵ������ | ʵ����� |
| ��ȡ�����������Թ��У���ˮ��ֱ����ȫ�ܽ� | | ����I������ |
| ��ȡ�����ٵ���Һ���Թ��У��μ�CaCl2��Һ������ | ֤����Na2CO3���� | |
| �� | ֤����NaOH���� | |
| �ۺ�����ʵ������˵������II�dz����ģ� | ||
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
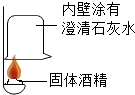 ��һ���ò��У�ͬѧ�Ƕ�ȼ�ϡ�����ƾ��������˺��棬���¶���ɷֽ����о���
��һ���ò��У�ͬѧ�Ƕ�ȼ�ϡ�����ƾ��������˺��棬���¶���ɷֽ����о���| ���� | 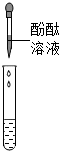 | 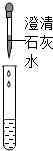 |
| ���� | ��Һ��� | ���� |
| ���� | ��Һ������������ | ��Һ����̼���� |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
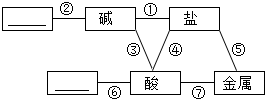 ������ѧϰ������Ҫ�������ܽ����ͼ��ʾ������֮��ķ�Ӧ��ϵ��ͼ�ж������ӵ����ʱ�ʾ�����Ӧ���������������пհף�
������ѧϰ������Ҫ�������ܽ����ͼ��ʾ������֮��ķ�Ӧ��ϵ��ͼ�ж������ӵ����ʱ�ʾ�����Ӧ���������������пհף��鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
| A������Ŵ�� |
| B������� |
| C��ǿ�������� |
| D����ȩ���ݹ�����Ҷ |
�鿴�𰸺ͽ���>>
����ʡ������Υ���Ͳ�����Ϣ�ٱ�ƽ̨ | �����к���Ϣ�ٱ�ר�� | ����թƭ�ٱ�ר�� | ����ʷ���������к���Ϣ�ٱ�ר�� | ������Ȩ�ٱ�ר��
Υ���Ͳ�����Ϣ�ٱ��绰��027-86699610 �ٱ����䣺58377363@163.com