�⣺��1����������������̼��������ɵģ��������ڻ����������
��2������ˮ��������ֽӴ�ʱ�������⣬����Һ�ܹ��ӿ�����������ʣ��������ˮ��ʳ��ˮ��ʳ��ʱ�����ĸ�ʴ���죮
��3������������ˮ��������ʱ���������⣮�������������ˮ����������
��4��������ϡ���ᷴӦ�Ļ�ѧ����ʽΪ��Fe
2O
3+6HCl�T2FeCl
3+3H
2O��
����ϡ���ᷴӦ�Ļ�ѧ����ʽΪ��Fe+2HCl�TFeCl
2+H
2����
��ʱ�䷴Ӧ����Һ���к�ɫ������������������̼���Ѹ����ʴ���Һ�з�������ķ����ǹ��ˣ����̼�����ˣ�
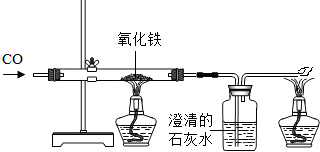
��5����������һ����̼��Ӧ�Ļ�ѧ����ʽΪ��Fe
2O
3+3CO

2Fe+3CO
2��
��6����������Ĵ������ޣ����Ҳ������������Էϸ���Ҫ�������ã�����������ã�
��7��ͭ�ڿ���������ͭ�̵Ļ�ѧ����ʽΪ��2Cu+H
2O+O
2+CO
2�TCu
2��OH��
2CO
3��
��8������ƽ�Ա���ƽ�⣬˵��ϡ������ȫ��Ӧ���������ĵ�������ʱ����Ӧ���������٣�����Ӧ����������������ƽʧȥƽ�⣬Ӧ����ϡ��������������ϡ���ᷴӦ���ɵ������٣�ָ��ƫ������һ�ߣ������������
�������ɶ���������ɵ��������ڻ�������������ˮ��ֽӴ�ʱ�������⣻���ݷ�Ӧ��������P�������غ㶨�ɿ�����д��ѧ����ʽ�����˿��Ѳ�����ˮ�����ʳ�ȥ�����������غ㶨�ɿ����жϷ�Ӧ���з���������
�����������Ҫ���ջ�ѧ����ʽ����д�����ͽ�������������ȷ����֪ʶ��ֻ���������ܶ���ط��������������ȷ���жϣ�


 2Fe+3CO2��
2Fe+3CO2��



 ��������Ҫ�Ľ������ϣ��ڽ��컴����ˮ�ɻ���ʱ�������˴����ĸ�����
��������Ҫ�Ľ������ϣ��ڽ��컴����ˮ�ɻ���ʱ�������˴����ĸ�����