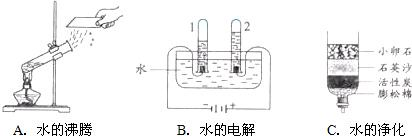
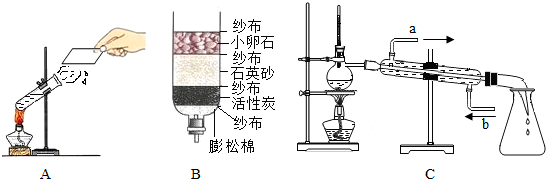
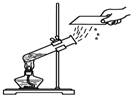
��1����ͼ��ʾ��3��ʵ�飬A��ˮ������
����
����
�����������ѧ�����仯��B���Թ�2�ڵõ�������Ϊ
������O2
������O2
����ʵ��˵��ˮ����
�⡢��Ԫ��
�⡢��Ԫ��
��ɵģ��÷�Ӧ�Ļ�ѧ����ʽ��
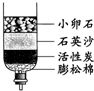
��C��С��ʯ��ʯӢɳ��������������
����
����
�������˾�ˮ���õ���ˮ��Ȼ���Ǵ�ˮ������õ���ˮ�ɲ��õķ�����
����
����
��
 |
 |
 |
| A��ˮ�ķ��� |
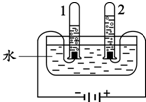
B��ˮ�ĵ�� |
C��ˮ�ľ��� |
��2����Ȫˮ������ˮ�ж������ã����磺þ���Ļ��Ϸ�Ӧʮ�ֻ��������μ�����ˮ����Ӧ�������ҽ��У���Ӧ�ٶȴ����ߣ���ʱˮ��������
������
������
��
��3����Լ��ˮ��ÿ�����������������ˮ��ʽӦ���ᳫ����
ACD
ACD
������ĸ��ţ���
A�������ڱ���ˮˢ����B������ϵر���ˮ��ϴ��
C��������ˮ��ϴ��ˮ�������������D������ࡢ�ι�ķ�������ũ�����
��4��ClO
2����һ������ˮ����������������������Cl
2��������ˮ����������ȡClO
2�ķ�Ӧ����ʾ��ͼ���£���ش�
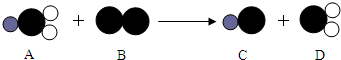
������

��ʾ��ԭ�ӣ�

��ʾ��ԭ�ӣ�

��ʾ��ԭ�ӣ�C��������Ԫ�صĻ��ϼ���
-1
-1
��D���ʵ�������
��������
��������
���÷�Ӧ�Ļ�ѧ����ʽ��
2NaClO2+Cl2�T2NaCl+2ClO2
2NaClO2+Cl2�T2NaCl+2ClO2
��������C��D������֮��
13��15
13��15
��





 ��ʾ��ԭ�ӣ�
��ʾ��ԭ�ӣ� ��ʾ��ԭ�ӣ�
��ʾ��ԭ�ӣ� ��ʾ��ԭ�ӣ�C��������Ԫ�صĻ��ϼ���
��ʾ��ԭ�ӣ�C��������Ԫ�صĻ��ϼ��� ��ʾ��ԭ�ӣ�
��ʾ��ԭ�ӣ� ��ʾ��ԭ�ӣ����֪D���ʵ����ƣ����Ʋ�A��B��C��D�Ļ�ѧʽ������д�÷�Ӧ�Ļ�ѧ����ʽ�����ݻ�ѧ����ʽ������ʵ������ȣ�
��ʾ��ԭ�ӣ����֪D���ʵ����ƣ����Ʋ�A��B��C��D�Ļ�ѧʽ������д�÷�Ӧ�Ļ�ѧ����ʽ�����ݻ�ѧ����ʽ������ʵ������ȣ�