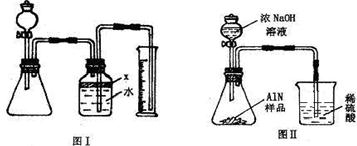
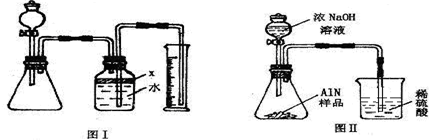
”¾“š°ø”æ
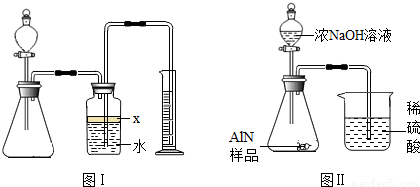
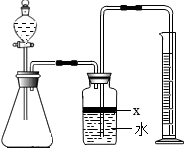
·ÖĪö£ŗ£Ø1£©ÖĘČ”ĘųĢåŹ±£¬ĪŖ·ĄÖ¹×°ÖĆĀ©ĘųÓ¦ŌŚĮ¬½Ó×°ÖĆŗóĮ¢¼“½ųŠŠ×°ÖĆĘųĆÜŠŌ¼ģ²é£¬Č·¶Ø×°ÖĆ²»Ā©Ęųŗ󣬱¾×ÅĻČ¼Ó¹ĢĢåŗó¼ÓŅŗĢåµÄŌŌņ¼ÓČėŅ©Ę·£»×īŗó½ųŠŠĘųĢåµÄŹÕ¼ÆÓė²āĮ棻
£Ø2£©¹Ų±Õ·ÖŅŗĀ©¶·µÄ»īČū£¬×°ÖĆÄŚŠĪ³É·ā±Õ»·¾³£¬Čē¹ū¶Ō×°ÖĆ½ųŠŠ¼ÓČČ£¬×°ÖĆÄŚĘųĢåŹÜČČĢå»ż±ä“ó£¬ČōĘųĆÜŠŌĮ¼ŗĆ£¬¾Ķ»į¹Ū²ģµ½¹ćæŚĘæÖŠÓŅ²ąµ¼¹ÜĖ®ÖłÉĻÉż£¬ŗćĪĀŹ±Ė®Öł²¢²»»ŲĀ䣻
£Ø3£©²śÉśµÄ°±Ęų¼«Ņ×ČÜÓŚĖ®£¬ĪŖ·ĄÖ¹°±ĘųČÜÓŚĖ®ŠčŅŖ°ŃĘųĢåÓėĖ®øōĄė£¬Ņņ“ĖӦєŌń²»ÄÜÓė°±Ęų²śÉś×÷ÓƵÄŅŗĢå×÷ĪŖøōĄėŅŗ£»
£Ø4£©±¾“ĪŹµŃéµÄÄæµÄŌŚÓŚ²ā¶Ø²śÉśĘųĢåµÄĢå»ż¶ų²»ŹĒŹÕ¼Æ“æ¾»µÄĘųĢ壬Ņņ“Ė£¬¹ćæŚĘæÄŚµÄŌÓŠĘųĢå²»ŌŚ²āĮæ·¶Ī§ÄŚ£»
£Ø5£©µŖ»ÆĀĮ”¢Ńõ»ÆĀĮ¶¼æÉÓėĒāŃõ»ÆÄĘČÜŅŗ·“Ó¦£¬ŠĪ³ÉĘ«ĀĮĖįÄĘČÜŅŗ”¢Ė®ŗĶ°±Ęų£¬³ä·Ö·“Ó¦ŗó²»»įÓŠ¹ĢĢåĪļÖŹµÄ²ŠĮō£»¶ųĢ¼²»ÄÜÓėĒāŃõ»ÆÄĘČÜŅŗ·“Ó¦£»
£Ø6£©ŃłĘ·ÖŠAlNµÄÖŹĮæ·ÖŹż=
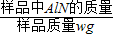
×100%£¬Ņņ“Ė£¬ŠčŅŖøł¾Ż·“Ó¦µÄ»Æѧ·½³ĢŹ½£¬ÓɲśÉś°±ĘųµÄÖŹĮæ¼ĘĖć²Ī¼Ó·“Ó¦µŖ»ÆĀĮµÄÖŹĮ棻
£Ø7£©°±ĘųÄÜÓėĮņĖį·“Ӧɜ³ÉĮņĖįļ§¶ųŹ¹Ļ”ĮņĖįČÜŅŗÖŹĮæŌö¼Ó£¬µ«ÓÉÓŚ·“Ó¦½ĻĪŖ¾ēĮŅ¶ų»įŹ¹Ļ”ĮņĖįµ¹Īü£¬¶ųŌģ³ÉÉÕ±ÄŚÖŹĮæ²»×¼Č·£»ĪŖ±ÜĆāøĆĻÖĻó³öĻÖ£¬æÉŌŚµ¼¹ÜÄ©¶Ė°²×°Ā©¶··ĄÖ¹µ¹Īü£»
£Ø8£©¢ŁĒāŃõ»ÆÄĘČÜŅŗ³Ź¼īŠŌ£¬æÉŹ¹ĪŽÉ«·ÓĢŖ±äŗģ£»ĒāŃõ»ÆÄĘÓėĮņĖįĆ¾ČÜŅŗ·“Ó¦£¬Éś³ÉĒāŃõ»ÆĆ¾³ĮµķŗĶĮņĖįÄĘČÜŅŗ£¬ĮņĖįÄĘČÜŅŗ³ŹÖŠŠŌ£¬µ±Ē”ŗĆĶźČ«·“Ó¦Ź±ĖłµĆČÜŅŗ²»ÄÜŹ¹·ÓĢŖ±äŗģ¶ų³ŹĪŽÉ«£»øł¾Ż·“Ó¦µÄ»Æѧ·½³ĢŹ½£¬ÓÉĒāŃõ»ÆÄʵÄÖŹĮææɼĘĖćĒ”ŗĆĶźČ«·“Ó¦Ź±Ėł¼ÓĮņĖįĆ¾µÄÖŹĮ棻øł¾ŻÖŹĮæŹŲŗć¶ØĀÉ£¬Ėł¼ÓĮņĖįĆ¾ČÜŅŗµÄÖŹĮæ=·“Ó¦ŗóČÜŅŗÖŹĮæ+ĒāŃõ»ÆĆ¾³ĮµķÖŹĮæ-ĒāŃõ»ÆÄĘČÜŅŗÖŹĮ棻
¢ŚĒāŃõ»ÆÄĘÓėĮņĖįĆ¾·“Ӧɜ³ÉĒāŃõ»ÆĆ¾³ĮµķŗĶĮņĖįÄĘ£¬ČÜŅŗÖŠÄĘĄė×ÓŹżÄæ²»±ä£¬¶ųĖęĮņĖįĆ¾µÄµĪ¼ÓĮņĖįøłĄė×Ó²»¶ĻŌö¶ą”¢ĒāŃõøłĄė×Ó²»¶Ļ¼õÉŁ£¬ŌČÜŅŗ²»ŗ¬Ć¾Ąė×ÓĒŅµĪČėĮņĖįĆ¾ŗóĀķÉĻÉś³ÉĒāŃõ»ÆĆ¾³Įµķ£¬ĖłŅŌµĪ¼ÓĒ°ŗóČÜŅŗÖŠŹ¼ÖÕ²»ŗ¬Ć¾Ąė×Ó£®
½ā“š£ŗ½ā£ŗ£Ø1£©Ó¦ĻČ½ųŠŠ×°ÖĆĘųĆÜŠŌ¼ģŃé£¬Č»ŗóŅĄ“Ī¼ÓČė¹ĢĢåŅ©Ę·”¢ŅŗĢåŅ©Ę·£¬×īŗó½ųŠŠĘųĢåÅųöĖ®µÄ²āĮæ£¬Č·¶Ø²śÉśĘųĢåĢå»ż£»
¹Ź“š°øĪŖ£ŗcabd£»
£Ø2£©Ķعż¼ÓČČ×°ÖĆÄŚĘųĢåŹ¹ĘųĢåĢå»ż±ä“ó£¬Čē¹ū×°ÖĆĀ©ĘųŌņ²»»į¹Ū²ģµ½×°ÖĆÄŚÓŠĆ÷ĻŌ±ä»Æ£»Čē¹ūĘųĆÜŠŌĮ¼ŗĆ£¬¹ćæŚĘæÖŠÓŅ²ąµ¼¹ÜĖ®ÖłÉĻÉż£¬ŗćĪĀŹ±Ė®Öł²¢²»»Ų£»
¹Ź“š°øĪŖ£ŗ¹Ų±Õ·ÖŅŗĀ©¶·»īČū£¬Ī¢ČČ׶ŠĪĘ棬¹ćæŚĘæÖŠÓŅ²ąµ¼¹ÜĖ®ÖłÉĻÉż£¬ŗćĪĀŹ±Ė®Öł²¢²»»ŲĀ䣻
£Ø3£©¾Ę¾«”¢ĘūÓĶŗĶĖÄĀČ»ÆĢ¼ĖäČ»¶¼²»ÄÜÓė°±Ęų·¢Éś·“Ó¦£¬µ«ĖüĆĒČ“¶¼¼«Ņ×»Ó·¢£¬»Ó·¢³öĄ“µÄĘųĢå¶ŌŹµŃéÓŠÓ°Ļģ¶ųĒŅ»Ó·¢Ķźŗó²»ÄÜŌŁĘšµ½øōĄė°±ĘųÓėĖ®½Ó“„µÄ×÷ÓĆ£»ŌŁ¼ÓÖ®¾Ę¾«Ņ×ČÜÓŚĖ®ĖÄĀČ»ÆĢ¼ĆܶȱČĖ®“󣬶¼²»ÄÜ“ļµ½øōĄėµÄÄæµÄ£»¶ųÖ²ĪļÓĶ¼Č²»ČÜÓŚĖ®Ņ²²»»Ó·¢£¬æÉŅŌ°Ń°±ĘųÓėĖ®½ųŠŠøōĄė£»
¹Ź“š°øĪŖ£ŗC£»
£Ø4£©±¾“ĪŹµŃéµÄÄæµÄŌŚÓŚ²ā¶Ø²śÉśĘųĢåµÄĢå»ż¶ų²»ŹĒŹÕ¼Æ“æ¾»µÄĘųĢ壬Ņņ“Ė£¬¹ćæŚĘæÄŚµÄŌÓŠĘųĢå²»ŌŚ²āĮæÄŚ£¬²»»į¶Ō²āĮæ½į¹ū²śÉśÓ°Ļģ£»
¹Ź“š°øĪŖ£ŗ²»±ä£»
£Ø5£©ÓÉÓŚĢ¼²»ÄÜÓėĒāŃõ»ÆÄĘČÜŅŗ·¢Éś·“Ó¦£¬¶ųµŖ»ÆĀĮ”¢Ńõ»ÆĀĮµČÓöĒāŃõ»ÆÄĘČÜŅŗ¶¼ÄÜČܽā¶ųĻūŹ§£¬Ņņ“Ė£¬æ“µ½ÓŠ¹ĢĢ岊ĮōŹ±£¬ĖµĆ÷Ō¹ĢĢåÖŠŗ¬ÓŠĢ¼£»
¹Ź“š°øĪŖ£ŗĢ¼£»
£Ø6£©ÉčµŖ»ÆĀĮµÄÖŹĮæĪŖx
AlN+NaOH+H
2O=NaAlO
2+NH
3ӟ
41 17
y

×17g
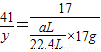
y=
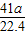
g
ѳʷ֊AlNµÄÖŹĮæ·ÖŹż=
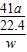
×100%=
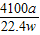
%
¹Ź“š°øĪŖ£ŗ
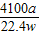
%£»
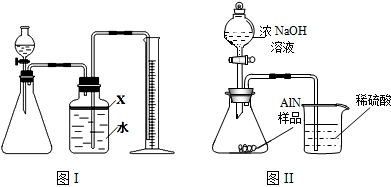
£Ø7£©°±Ęų¼«Ņ×ČÜÓŚĻ”ĮņĖį¶ų³öĻÖµ¹Īü£¬Ņņ“Ė£¬øĆ×°ÖĆ²»ÄÜ×¼Č·²āĮæ²śÉś°±ĘųµÄĮ棻æÉŌŚµ¼¹ÜÄ©¶ĖĮ¬½ÓĀ©¶·µ¹æŪŌŚŅŗĆęÉĻ£¬øÕ°±Ęų“óĮæĪüŹÕŹ±£¬ÉÕ±ÄŚŅŗĆęĻĀ½µ¶ųĶŃĄė½Ó“„£¬æÉŅŌ·ĄÖ¹Ļ”ĮņĖįµÄµ¹Īü£»
¹Ź“š°øĪŖ£ŗ²»æÉŠŠ£»°±Ęų¼«Ņ×±»ĪüŹÕ£¬·¢Éśµ¹ĪüĻÖĻó£»ÉÕ±µ¼¹ÜµÄÄ©¶Ė½ÓŅ»µ¹æŪµÄĀ©¶·Ą“ĪüŹÕ°±Ęų£Ø»ņĘäĖüŗĻĄķ“š°ø£©£»
£Ø8£©¢ŁÉč£ŗĮņĖįĆ¾µÄÖŹĮæĪŖx£¬Éś³É³ĮµķÖŹĮæĪŖy£®
2NaOH+MgSO
4ØTNa
2SO
4+Mg£ØOH£©
2”ż
80 120 142 58
0.2m x y
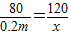
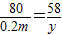
½āµĆ£ŗx=0.3m y=0.145m
Ėł¼ÓĮņĖįĆ¾ČÜŅŗµÄÖŹĮæ·ÖŹż=
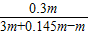
×100%=14.0%
“š£ŗĖł¼ÓĮņĖįĆ¾ČÜŅŗµÄÖŹĮæ·ÖŹżĪŖ14.0%£»
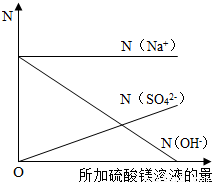
¢Ś·“Ó¦Ē°ŗóČÜŅŗÖŠÄĘĄė×ÓŹżÄæ²»±ä£¬¶ųĒāŃõøłĄė×ÓĖęĮņĖįĆ¾µÄµĪ¼Ó²»¶ĻÉś³ÉĒāŃõ»ÆĆ¾³Įµķ¶ų¼õÉŁ£»Ėę×ÅĮņĖįĆ¾µÄµĪ¼Ó£¬ČÜŅŗÖŠµÄĮņĖįøłĄė×Ó²»¶ĻŌö¼Ó£»ČÜŅŗÖŠŹ¼ÖÕ²»ŗ¬Ć¾Ąė×Ó£»
¹Ź“š°øĪŖ£ŗČēĶ¼ĖłŹ¾
 µćĘĄ£ŗ
µćĘĄ£ŗĄūÓĆ»Æѧ±ä»ÆĒ°ŗóŌŖĖŲÖÖĄąŗĶÖŹĮæ²»±ä£¬æÉĄūÓĆ°±ĘųÖŠNŌŖĖŲµÄÖŹĮæ¼ĘĖćѳʷ֊µŖ»ÆĀĮµÄÖŹĮ森

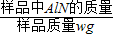 ×100%£¬Ņņ“Ė£¬ŠčŅŖøł¾Ż·“Ó¦µÄ»Æѧ·½³ĢŹ½£¬ÓɲśÉś°±ĘųµÄÖŹĮæ¼ĘĖć²Ī¼Ó·“Ó¦µŖ»ÆĀĮµÄÖŹĮ棻
×100%£¬Ņņ“Ė£¬ŠčŅŖøł¾Ż·“Ó¦µÄ»Æѧ·½³ĢŹ½£¬ÓɲśÉś°±ĘųµÄÖŹĮæ¼ĘĖć²Ī¼Ó·“Ó¦µŖ»ÆĀĮµÄÖŹĮ棻 ×17g
×17g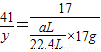
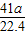 g
g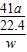 ×100%=
×100%=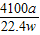 %
%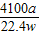 %£»
%£»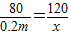
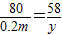
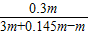 ×100%=14.0%
×100%=14.0%


 ĘŚÄ©¼Æ½įŗÅĻµĮŠ“š°ø
ĘŚÄ©¼Æ½įŗÅĻµĮŠ“š°ø
 µŖ»ÆĀĮ£ØAlN£©ŹĒŅ»ÖÖŠĀŠĶĪŽ»ś²ÄĮĻ£¬¹ć·ŗÓ¦ÓĆÓŚ¼Æ³ÉµēĀ·Éś²śĮģÓņ£®Ä³µŖ»ÆĀĮÖŠŗ¬ÓŠĢ¼»ņŃõ»ÆĀĮŌÓÖŹ£¬Ä³»Æѧъ¾æŠŌѧĻ°Š”×éÉč¼Ę²¢Ķź³ÉĮĖČēĻĀŹµŃ飮
µŖ»ÆĀĮ£ØAlN£©ŹĒŅ»ÖÖŠĀŠĶĪŽ»ś²ÄĮĻ£¬¹ć·ŗÓ¦ÓĆÓŚ¼Æ³ÉµēĀ·Éś²śĮģÓņ£®Ä³µŖ»ÆĀĮÖŠŗ¬ÓŠĢ¼»ņŃõ»ÆĀĮŌÓÖŹ£¬Ä³»Æѧъ¾æŠŌѧĻ°Š”×éÉč¼Ę²¢Ķź³ÉĮĖČēĻĀŹµŃ飮