
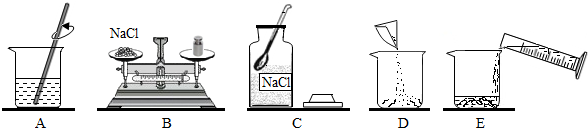
���� ��1�������������������Ĺ�ʽ���м��㣻
��2���������Ƹ���Һ�IJ����У����㡢�������ܽ⡢װƿ�Ƚ��з�����
��3������������ƽ����ҩƷ�ķ������з�����
��4�������������Ĺ���NaCl����������ɳ����ʹ�Ȼ����������ٽ��з�����
��� �⣺��1������80g������������Ϊ15%���Ȼ�����Һ���裺�Ȼ��Ƶ�����Ϊ��80g��15%=12g��ˮ�������ǣ�80g-12g=68g��
��2�����Ƹ���Һ�IJ����У����㡢�������ܽ⡢װƿ��������ȷ���Ƹ���Һ�IJ���˳��Ϊ��CBDEA��
��3����������ƽ����������Ȼ���ʱ������������ƽ��ָ��ƫ�����̣�˵�������ʳ�ε��������࣬Ӧ���������Ȼ��ƹ��壬��ѡ��B��
��4���������Ĺ���NaCl����������ɳ����ʹ�Ȼ����������٣��ܼ��������䣬���������Ƶ���Һ��������������15%��
�ʴ�Ϊ����1��12g��68g��
��2��CBDEA��
��3��B��
��4������
���� �����Ϊ����Ϥ�������������Ĺ�ʽ����ȷʹ��������ƽ�ǽ����Ļ�����


 ��У����ϵ�д�
��У����ϵ�д�
| �꼶 | ���пγ� | �꼶 | ���пγ� |
| ��һ | ��һ��ѿγ��Ƽ��� | ��һ | ��һ��ѿγ��Ƽ��� |
| �߶� | �߶���ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
| ���� | ������ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
��Ŀ�����л�ѧ ��Դ�� ���ͣ������
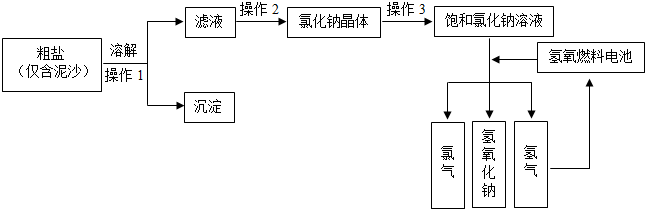
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�ѡ����
| A�� | ������-Na2SO4 | B�� | ��ʯ��-CaO | C�� | ̼����-Na2CO3 | D�� | �ɱ�--H2O |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�ѡ����
 �����ܽ��������ͼ�������й�˵����ȷ���ǣ�������
�����ܽ��������ͼ�������й�˵����ȷ���ǣ�������| A�� | t2��ʱa��c �������ܽ����� | |
| B�� | ��c�IJ�������Һ���¿���ת��Ϊ������Һ | |
| C�� | t1��ʱa��c������Һ����������������� | |
| D�� | ��t3���a��b��c �������ʵı�����Һ��ȴ��t1 ʱ��a����������࣬��c�������� |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ������

�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ������
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ������

�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ������
�鿴�𰸺ͽ���>>
����ʡ������Υ���Ͳ�����Ϣ�ٱ�ƽ̨ | �����к���Ϣ�ٱ�ר�� | ����թƭ�ٱ�ר�� | ����ʷ���������к���Ϣ�ٱ�ר�� | ������Ȩ�ٱ�ר��
Υ���Ͳ�����Ϣ�ٱ��绰��027-86699610 �ٱ����䣺58377363@163.com