
 Ä³Ę·ÅĘŅūÓĆĢģČ»Ė®µÄ¹ćøęÖŠÓŠČēĻĀ»Ćę£ŗĮ½øö²£Į§ÖŠ·Ö±šŹ¢ÓŠĮ½ÖÖĖ®Ńł£¬ĘäÖŠŅ»Ö»Ź¢øĆĘ·ÅĘŅūÓĆĢģČ»Ė®£¬ĮķŅ»Ö»Ź¢ĘäĖūµÄĖ®£®Ė®ŃłÖŠ·Ö±š½žÓŠpHŹŌÖ½ŗó£¬Ņ»±³ŹĻֵ飻ĘÉ«£ØČõĖįŠŌ£©£¬Ņ»±³ŹĻÖµĀĢÉ«£ØČõ¼īŠŌ£©£¬¹ćøęĢįŹ¾“ó¼Ņ£ŗČõ¼īŠŌµÄĖ®¶ŌČĖĢå½”æµÓŠĄū£®Ēė»Ų“š£ŗ
Ä³Ę·ÅĘŅūÓĆĢģČ»Ė®µÄ¹ćøęÖŠÓŠČēĻĀ»Ćę£ŗĮ½øö²£Į§ÖŠ·Ö±šŹ¢ÓŠĮ½ÖÖĖ®Ńł£¬ĘäÖŠŅ»Ö»Ź¢øĆĘ·ÅĘŅūÓĆĢģČ»Ė®£¬ĮķŅ»Ö»Ź¢ĘäĖūµÄĖ®£®Ė®ŃłÖŠ·Ö±š½žÓŠpHŹŌÖ½ŗó£¬Ņ»±³ŹĻֵ飻ĘÉ«£ØČõĖįŠŌ£©£¬Ņ»±³ŹĻÖµĀĢÉ«£ØČõ¼īŠŌ£©£¬¹ćøęĢįŹ¾“ó¼Ņ£ŗČõ¼īŠŌµÄĖ®¶ŌČĖĢå½”æµÓŠĄū£®Ēė»Ų“š£ŗ·ÖĪö £Ø1£©øł¾ŻĢāŅā£¬Čõ¼īŠŌµÄĖ®¶ŌČĖĢå½”æµÓŠĄū£¬½ųŠŠ·ÖĪö½ā“š£®
£Ø2£©øł¾ŻÓĆpHŹŌÖ½²ā¶ØĪ“ÖŖČÜŅŗµÄpHµÄ·½·Ø£¬½ųŠŠ·ÖĪö½ā“š£®
£Ø3£©pHŹŌÖ½ÉĻµÄ±ź×¼±ČÉ«æØÉĻµÄŹż×ÖÖ»ÓŠÕūŹż£¬¼“Ź¹ÓĆpHŹŌÖ½Ėł²āµĆµÄČÜŅŗĖį¼ī¶ČĪŖÕūŹż£®
½ā“š ½ā£ŗ£Ø1£©ÓÉĢāŅā£¬Čõ¼īŠŌµÄĖ®¶ŌČĖĢå½”æµÓŠĄū£¬Ōņ³£ĪĀŹ±£¬øĆĘ·ÅĘŅūÓĆĢģČ»Ė®Ó¦³ŹČõ¼īŠŌ£¬pHæÉÄÜĪŖ7.3£®
£Ø2£©ÓĆpHŹŌÖ½²ā¶ØĪ“ÖŖČÜŅŗµÄpHŹ±£¬ÕżČ·µÄ²Ł×÷·½·ØĪŖÓĆ²£Į§°ōÕŗȔɣĮæ“ż²āŅŗµĪŌŚøÉŌļµÄpHŹŌÖ½ÉĻ£¬Óė±ź×¼±ČÉ«æضŌ±ČĄ“Č·¶ØpH£®²»Äܽ«pHŹŌÖ½ÉģČė“ż²āŅŗÖŠ£¬ŅŌĆāĪŪČ¾“ż²āŅŗ£¬Ķ¼Ź¾ÖŠ²ā¶ØĖ®ŃłpHµÄ·½·Ø£¬²»Äܽ«pHŹŌÖ½Ö±½Ó·ÅČė²£Į§±µÄĖ®ŃłÖŠ£®
£Ø3£©pHŹŌÖ½ÉĻµÄ±ź×¼±ČÉ«æØÉĻµÄŹż×ÖÖ»ÓŠÕūŹż£¬¼“Ź¹ÓĆpHŹŌÖ½Ėł²āµĆµÄČÜŅŗĖį¼ī¶ČĪŖÕūŹż£¬“Ó£Ø1£©ÖŠŃ”³öµÄ½į¹ū²»ÄÜÓĆŅ»°ćŹµŃéŹŅµÄpHŹŌÖ½²ā³ö£®
¹Ź“š°øĪŖ£ŗ£Ø1£©C£»£Ø2£©½«pHŹŌÖ½Ö±½Ó·ÅČė²£Į§±µÄĖ®ŃłÖŠ£»£Ø3£©²»ÄÜ£®
µćĘĄ ±¾ĢāÄŃ¶Č²»“ó£¬ÕĘĪÕÓĆpHŹŌÖ½²ā¶ØĪ“ÖŖČÜŅŗµÄpHµÄ·½·Ø”¢×¢ŅāŹĀĻī£Ø²»ÄܽžČė“ż²āŅŗ”¢²»ÄÜÓĆĖ®ČóŹŖ£©ŹĒÕżČ·½ā“š±¾ĢāµÄ¹Ų¼ü£®


 ĢģĢģĻņÉĻŅ»±¾ŗĆ¾ķĻµĮŠ“š°ø
ĢģĢģĻņÉĻŅ»±¾ŗĆ¾ķĻµĮŠ“š°ø Š”ѧɜ10·ÖÖÓÓ¦ÓĆĢāĻµĮŠ“š°ø
Š”ѧɜ10·ÖÖÓÓ¦ÓĆĢāĻµĮŠ“š°ø
| Äź¼¶ | øßÖŠæĪ³Ģ | Äź¼¶ | ³õÖŠæĪ³Ģ |
| øßŅ» | øßŅ»Ćā·ŃæĪ³ĢĶĘ¼ö£” | ³õŅ» | ³õŅ»Ćā·ŃæĪ³ĢĶĘ¼ö£” |
| ø߶ž | ø߶žĆā·ŃæĪ³ĢĶĘ¼ö£” | ³õ¶ž | ³õ¶žĆā·ŃæĪ³ĢĶĘ¼ö£” |
| øßČż | øßČżĆā·ŃæĪ³ĢĶĘ¼ö£” | ³õČż | ³õČżĆā·ŃæĪ³ĢĶĘ¼ö£” |
æĘÄæ£ŗ³õÖŠ»Æѧ Ą“Ō“£ŗ ĢāŠĶ£ŗŃ”ŌńĢā
| A£® | 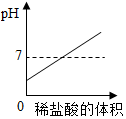 | B£® |  | C£® |  | D£® |  |
²éæ““š°øŗĶ½āĪö>>
æĘÄæ£ŗ³õÖŠ»Æѧ Ą“Ō“£ŗ ĢāŠĶ£ŗŹµŃéĢ½¾æĢā
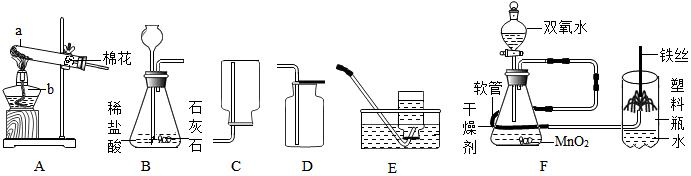
²éæ““š°øŗĶ½āĪö>>
æĘÄæ£ŗ³õÖŠ»Æѧ Ą“Ō“£ŗ ĢāŠĶ£ŗŃ”ŌńĢā
| A£® | ÉśŠāµÄĢś¶¤ÓėŃĪĖį·“Ó¦ŗóæɵƵ½ĀČ»ÆĢśČÜŅŗ | |
| B£® | ×ĻÉ«ŹÆČļŹŌŅŗÓöµ½Ėį±äŗģÉ« | |
| C£® | ĒāŃõ»ÆĶæÉŅŌČܽāŌŚĻ”ĮņĖįÖŠ | |
| D£® | ČĪŗĪĖį¶¼ÄÜÓėĀČ»Æ±µČÜŅŗ·“Ӧɜ³É°×É«³Įµķ |
²éæ““š°øŗĶ½āĪö>>
æĘÄæ£ŗ³õÖŠ»Æѧ Ą“Ō“£ŗ ĢāŠĶ£ŗŃ”ŌńĢā
| A£® | ľéČ»ØµÄČÜŅŗÓėĖį×÷ÓĆĻŌŗģÉ«ŹĒ»Æѧ±ä»Æ | |
| B£® | ÄÜŹ¹Ä¾éČ»ØµÄČÜŅŗĻŌĀĢÉ«µÄŅ»¶ØŹĒ¼ī | |
| C£® | ½«ĀČ»ÆÄĘČÜŅŗµĪČėľéČ»ØµÄČÜŅŗŗó£¬ČÜŅŗČŌĪŖ×ĻÉ« | |
| D£® | ľéČ»ØµÄČÜŅŗæÉÓĆ×÷Ėį¼īÖøŹ¾¼Į |
²éæ““š°øŗĶ½āĪö>>
æĘÄæ£ŗ³õÖŠ»Æѧ Ą“Ō“£ŗ ĢāŠĶ£ŗŃ”ŌńĢā
| A£® | ŃĪĖįÓöµ½×ĻÉ«ŹÆČļŹŌŅŗ±äŗģÉ« | |
| B£® | ŃĪĖįŹĒĀČ»ÆĒāĘųĢåµÄĖ®ČÜŅŗ | |
| C£® | ŃĪĖįÄÜŹ¹ĪŽÉ«·ÓĢŖŹŌŅŗ±äŗģ | |
| D£® | ÓĆ¼ÓČČÕō·¢ČܼĮµÄ·½·Ø¾ĶæÉŹ¹Ļ”ŃĪĖį±ä³ÉÅØŃĪĖį |
²éæ““š°øŗĶ½āĪö>>
æĘÄæ£ŗ³õÖŠ»Æѧ Ą“Ō“£ŗ ĢāŠĶ£ŗĢīæÕĢā
²éæ““š°øŗĶ½āĪö>>
æĘÄæ£ŗ³õÖŠ»Æѧ Ą“Ō“£ŗ ĢāŠĶ£ŗĢīæÕĢā
²éæ““š°øŗĶ½āĪö>>
æĘÄæ£ŗ³õÖŠ»Æѧ Ą“Ō“£ŗ ĢāŠĶ£ŗŹµŃéĢ½¾æĢā
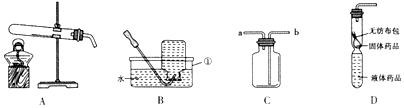
²éæ““š°øŗĶ½āĪö>>
°Ł¶ČÖĀŠÅ - Į·Ļ°²įĮŠ±ķ - ŹŌĢāĮŠ±ķ
ŗž±±Ź”»„ĮŖĶųĪ„·ØŗĶ²»Į¼ŠÅĻ¢¾Ł±ØĘ½ĢØ | ĶųÉĻÓŠŗ¦ŠÅĻ¢¾Ł±Ø×ØĒų | µēŠÅÕ©Ę¾Ł±Ø×ØĒų | É꥜Ź·ŠéĪŽÖ÷ŅåÓŠŗ¦ŠÅĻ¢¾Ł±Ø×ØĒų | ÉęĘóĒÖČؾŁ±Ø×ØĒų
Ī„·ØŗĶ²»Į¼ŠÅĻ¢¾Ł±Øµē»°£ŗ027-86699610 ¾Ł±ØÓŹĻä£ŗ58377363@163.com