
��9�֣�A~G���dz��л�ѧѧϰ���ij������ʣ���Щ������һ�������£�����������ͼ��ʾ��ת����ϵ����֪A��EΪ���ʣ�����Ϊ�������G�Ǵ���ʯ�е���Ҫ�ɷ֡�
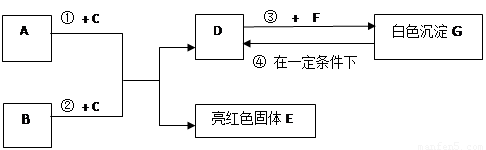 |
�����ͼʾ�Ʋ⣬�ش��������⣺
��1��B��C�Ļ�ѧʽ�ֱ��� �� ��
��2��F��ˮ��Һ�������������� ��
��3����Ӧ�� �Ļ�ѧ����ʽΪ_____________ ��
��4���ڳ����·����ķ�Ӧ�ܵĻ�ѧ����ʽΪ __________________ ��
��5��50g G��һ��������ת��ΪDʱ�����õ���D�������� g��
��1�� CO ��1�֣� CuO ��1�֣�
��2�� Ca2+ ��OH�C ��1�֣�
��3�� C + 2CuO  2CuO + CO2 �� ��2�֣�δ��ƽ��©������©�� ֻ��1�֣�
2CuO + CO2 �� ��2�֣�δ��ƽ��©������©�� ֻ��1�֣�
��4�� CaCO3 + 2HCl = CaCl2 + H2O + CO2 �� ��2�֣�δ��ƽ��©�� ֻ��1�֣�
��5�� 22 ��2�֣�
��������E������ɫ���嵥�ʣ�Ӧ���ǽ���ͭ�����ݰ�ɫ����G��һ�ֽ������ϣ������ƶ�GΪ̼��ƣ�DΪ������̼��C��CuO����ΪA�ǵ��ʣ�����AΪ̼���ʣ���ôBӦ��Ϊһ����̼��D��F��Ӧ������̼��ƣ���ôFӦ�����������ƣ����˸����ʼ�����ϣ����Ծݴ˴��⣮


 �Ķ��쳵ϵ�д�
�Ķ��쳵ϵ�д�
| �꼶 | ���пγ� | �꼶 | ���пγ� |
| ��һ | ��һ��ѿγ��Ƽ��� | ��һ | ��һ��ѿγ��Ƽ��� |
| �߶� | �߶���ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
| ���� | ������ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
��9�֣�A~G���dz��л�ѧѧϰ���ij������ʣ���Щ������һ�������£�����������ͼ��ʾ��ת����ϵ����֪A��EΪ���ʣ�����Ϊ�������G�Ǵ���ʯ�е���Ҫ�ɷ֡�
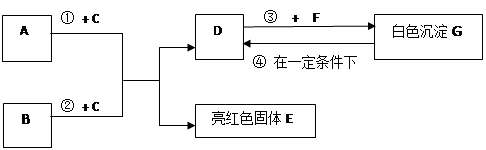 |
�����ͼʾ�Ʋ⣬�ش��������⣺
��1��B��C�Ļ�ѧʽ�ֱ��� �� ��
��2��F��ˮ��Һ�������������� ��
��3����Ӧ�� �Ļ�ѧ����ʽΪ_____________ ��
��4���ڳ����·����ķ�Ӧ�ܵĻ�ѧ����ʽΪ __________________ ��
��5��50g G��һ��������ת��ΪDʱ�����õ���D�������� g��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
 |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ��2012��㶫ʡ�����а������п�ģ�⻯ѧ�Ծ� ���������� ���ͣ��ƶ���
��9�֣�A~G���dz��л�ѧѧϰ���ij������ʣ���Щ������һ�������£�����������ͼ��ʾ��ת����ϵ����֪A��EΪ���ʣ�����Ϊ�������G�Ǵ���ʯ�е���Ҫ�ɷ֡�
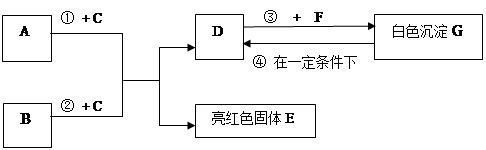 |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ��2011-2012ѧ��ɽ��ʡ���꼶�п�ģ�⣨������ѧ�Ծ��������棩 ���ͣ��ƶ���
A~G���dz��л�ѧѧϰ���ij������ʣ���Щ������һ�������£�����������ͼ��ʾ��ת����ϵ����֪A��EΪ���ʣ�����Ϊ�������G�Ǵ���ʯ�е���Ҫ�ɷ֡�
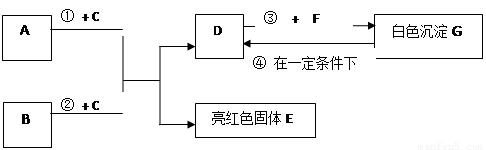
�����ͼʾ�Ʋ⣬�ش��������⣺
��1��B��C�Ļ�ѧʽ�ֱ��� �� ��
��2��F��ˮ��Һ�������������� ��
��3����Ӧ�� �Ļ�ѧ����ʽΪ_____________ ��
��4���ڳ����·����ķ�Ӧ�ܵĻ�ѧ����ʽΪ ____________ ��
��5��50g G��һ��������ת��ΪDʱ�����õ���D�������� g��
�鿴�𰸺ͽ���>>
����ʡ������Υ���Ͳ�����Ϣ�ٱ�ƽ̨ | �����к���Ϣ�ٱ�ר�� | ����թƭ�ٱ�ר�� | ����ʷ���������к���Ϣ�ٱ�ר�� | ������Ȩ�ٱ�ר��
Υ���Ͳ�����Ϣ�ٱ��绰��027-86699610 �ٱ����䣺58377363@163.com