
����Ŀ��ijС��ͬѧ��ʵ���ҽ���ʵ�飬������롣
��1��ͬѧ����ȡ�����ڿ����о��õ��������ƹ�����Ʒ��ˮ���Ƴ���Һ����ȡ2mL����Һ��С�ձ��У�Ȼ��μӼ��η�̪��Һ������ʱ�۲쵽��ʵ������Ϊ��_____________________���������ձ�����μ���ϡ���Ტ�ò��������裬��Һ����ɫ�ı䣬ͬʱ�۲쵽�����ݲ��������ۣ��������Ʊ��ʡ��뻯ѧ����ʽ�����������Ʊ��ʵ�ԭ��_____________________��
��2������������Ʊ��ʵij̶����˽�һ��̽����
���������룩����һ����Ʒ���ֱ��ʣ��ɷ�ΪNaOH��Na2CO3��
���������Ʒ��ȫ���ʣ��ɷ�Ϊ_______________��
���������ϣ���BaCl2��Һ�����ԣ�BaCO3������ˮ������̼������Һ����μ���ϡ����ķ�Ӧ�Ƿֲ���Ӧ���ᷢ������̼�����ƺ��Ȼ��Ƶķ�Ӧ��
��ʵ��̽����Ϊ��֤���룬ͬѧ�����������������������ʵ�顣
�ٶ��Է�����ƣ����㽫��ʵ����Ʋ���������
ʵ������ | ʵ������ | �ó����� |
ȡ������Ʒ��Һ����������BaCl2��Һ�����ã����ϲ���Һ�еμӼ�����ɫ��̪��Һ | ______________ | ����һ��ȷ |
�ڶ���������� ��ȷ��ȡ 11.95g ���ʵ�NaOH��Ʒ������ƿ�У��õ��ӳӳƵ���ƿ����Ʒ��������Ϊ46.95g���ٰ� 150.00 g7.3 ��ϡ����ƽ���ֳ� 6�ȷݣ�ÿ��25.00g�����ڳ��ҡ����ƿʱ��������Ʒ�У�ÿ�γ�ַ�Ӧ���õ��ӳӳƵ���ƿ����ʢ���ʵ�������ʵ�����ݼ�¼���£�
��������Ĵ��� | ��һ�� | �ڶ��� | ������ | ���Ĵ� | ����� | ������ |
��ƿ����ʢ����������/g | 71.95 | 96.95 | 120.85 | 144.75 | 168.65 | 193.65 |
��ʵ����ۣ����������ʵ�����ݣ�������Ʒ���������Ƶ���������Ϊ______����ȷ��0.1%��������һ��ȷ��
����˼������
������ͼ��ֱ������ϵ�л����������������������ϡ���������Ĺ�ϵ����______��
�����жϵڶ��μ�����������з�����Ӧ�Ļ�ѧ����ʽ_________________��ԭ��______________________��
���𰸡���Һ����ɫ��Ϊ��ɫ 2NaOH+CO2=Na2CO3+H2O Na2CO3 �а�ɫ�����������ϲ���Һ����ɫ��Ϊ��ɫ 33.5% 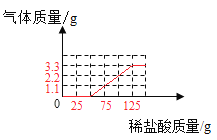 NaOH+HCl=NaCl+H2O ��Ϊʵ��������ܹ�����3.3g������̼��ͨ���������3.3g������̼������75gϡ���ᣬ��˵��������̼���Ƶķ�Ӧǡ�����ڵ�3��5�μ������ʵ������н��У������������������Ƶķ�Ӧ�����ڵ�1��2����ʵ����
NaOH+HCl=NaCl+H2O ��Ϊʵ��������ܹ�����3.3g������̼��ͨ���������3.3g������̼������75gϡ���ᣬ��˵��������̼���Ƶķ�Ӧǡ�����ڵ�3��5�μ������ʵ������н��У������������������Ƶķ�Ӧ�����ڵ�1��2����ʵ����
��������
��1����������������еĶ�����̼��Ӧ����̼���ƣ�̼���ƺ�����������Һ���Լ��ԣ�ȡ�����ڿ����о��õ��������ƹ�����Ʒ��ˮ���Ƴ���Һ����ȡ2mL����Һ��С�ձ��У�Ȼ��μӼ��η�̪��Һ������ʱ�۲쵽��ʵ������Ϊ����Һ����ɫ��Ϊ��ɫ���������ձ�����μ���ϡ���ᣬϡ�����̼���Ʒ�Ӧ�����Ȼ��ơ�ˮ�Ͷ�����̼���壬
�����Һ����ɫ��Ϊ��ɫ��2NaOH+CO2=Na2CO3+H2O��
[��������]����һ����Ʒ���ֱ��ʣ��ɷ�ΪNaOH��Na2CO3��
���������Ʒ��ȫ���ʣ��ɷ�ΪNa2CO3��
[ʵ��̽��]��
ʵ������ | ʵ������ | �ó����� |
ȡ������Ʒ��Һ����������BaCl2��Һ�����ã����ϲ���Һ�еμӼ�����ɫ��̪��Һ | �а�ɫ�����������ϲ���Һ����ɫ��Ϊ��ɫ | ����һ��ȷ |
���Na2CO3���а�ɫ�����������ϲ���Һ����ɫ��Ϊ��ɫ��
�ڵ���μ���ϡ����ʱ��ϡ�����̼���Ʒ�Ӧ�����ɶ�����̼������Ϊ46.95g+125g-168.65g=3.3g������Ʒ��̼���Ƶ�����Ϊx��
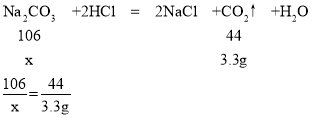
x=7.95g
��Ʒ���������Ƶ���������Ϊ![]() ��
��
���33.5%��
[��˼����]
�ٲ���������̼���������������ϡ���������Ĺ�ϵ������ͼ

��ʵ��������ܹ�����3.3g������̼��ͨ���������3.3g������̼������75gϡ���ᣬ��˵��������̼���Ƶķ�Ӧǡ�����ڵ�3��5�μ������ʵ������н��У������������������Ƶķ�Ӧ�����ڵ�1��2����ʵ���У�ϡ������������Ʒ�Ӧ�����Ȼ��ƺ�ˮ����ѧ����ʽΪNaOH+HCl=NaCl+H2O��
��� ��
��
NaOH+HCl=NaCl+H2O��ʵ��������ܹ�����3.3g������̼��ͨ���������3.3g������̼������75gϡ���ᣬ��˵��������̼���Ƶķ�Ӧǡ�����ڵ�3��5�μ������ʵ������н��У������������������Ƶķ�Ӧ�����ڵ�1��2����ʵ���С�


 ���ѵ����Ԫ��ĩ���100��ϵ�д�
���ѵ����Ԫ��ĩ���100��ϵ�д� ��˼άС�ھ�100����ҵ��ϵ�д�
��˼άС�ھ�100����ҵ��ϵ�д� ��ʦָ��һ��ͨϵ�д�
��ʦָ��һ��ͨϵ�д�
| �꼶 | ���пγ� | �꼶 | ���пγ� |
| ��һ | ��һ��ѿγ��Ƽ��� | ��һ | ��һ��ѿγ��Ƽ��� |
| �߶� | �߶���ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
| ���� | ������ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
����Ŀ����ͼ��һЩ���ʵ��ܽ�����ߡ�

��1����20��ʱ����ʢ��100gˮ���ձ��м���25gKNO3���裬�۲쵽�������� ������Һ�������� ��
��2������1�����ձ����ټ���25gKNO3���裬�� ��KNO3����û���ܽ⣬��ʱ���õ���Һ�� ��������������������������Һ��
��3���ڣ�2�����õ���Һ��KNO3�������� g��
��4��KNO3��Һ�к�������NaClʱ���ᴿ�ķ�����
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
����Ŀ���ס��ҡ���������Ϊ���л�ѧ�������ʣ�����֮��IJ���ת����ϵ��ͼ��ʾ�����ڸ�ת����ϵͼ������˵����ȷ���ǣ� ��
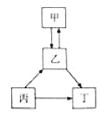
A���������̼���ƣ������������ƣ������������
B�����ס��ҡ���������������һ����̼���
C���ס��ҡ�������֮���ת������ȫ��ͨ�����ֽⷴӦʵ��
D�����ס��ҡ�������������ͬһ��Ԫ�أ����ҿ�����������һ���ǵ���̼
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
����Ŀ���������������ͭ�Ļ����Һ200 g�������Һ����μ�����������Ϊ10%������������Һֱ������Cu(OH)2���������������������������������Һ��������ϵ��ͼ��ʾ��
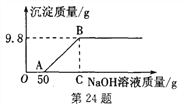
(1) Cu(OH)2����Է���������__________��
(2)������������ʱ�����������������Һ��������Ϊ________��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
����Ŀ���ס��ҡ����������ʼ��ת����ϵ��ͼ��ʾ����ס��ҡ�����˳������( )
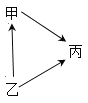
A.H2O ��H2O2 ��O2B.Ca(OH)2��CaCO3��CaO
C.Al2O3��Al��Al2(SO4)3D.CO��C��CO2
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
����Ŀ�������Ͷ�����̼��������Ҫ�����壬�����ѧ����֪ʶ����
�ٹ�ҵ���÷���Һ̬�����ķ�����ȡ��������Ҫ�������������͵�����_______��ͬ��������̼ͨ������ȼ��Ҳ����ȼ���ܶȴ��ڿ��������ͨ��������______________��
�ڸ��������������ش����⡣
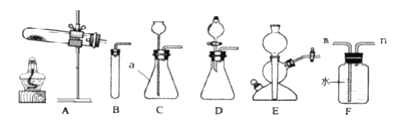
��.д������a���ƣ�a______��
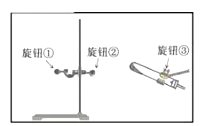
��.ijͬѧ��Aװ����ȡ����ʱ�����Թ�λ��̫�ߣ�������������ȣ���ͬѧӦ������ͼ�е���ť____���������������������������������ø������Aװ����ȡ��������Ӧ�Ļ�ѧ����ʽ��____________________������װ��F�ռ������Բ���ռ�![]() �����������____����m��n�������롣
�����������____����m��n�������롣
��.B��C��D��E������Ϊʵ�����Ʊ�![]() �ķ���װ�ã���C��ȣ�Bװ�õIJ���֮����__________________��ʹ��Eװ��ʱ��װ��E���ŵ���______________��Ҫʹ��Ӧֹͣ��Ӧ���еIJ�����_______________��
�ķ���װ�ã���C��ȣ�Bװ�õIJ���֮����__________________��ʹ��Eװ��ʱ��װ��E���ŵ���______________��Ҫʹ��Ӧֹͣ��Ӧ���еIJ�����_______________��
��.��ʯ��ʯ��������ϡ�����ַ�Ӧ���Ƶö�����̼Ϊ![]() ���Լ��㣺�μӷ�Ӧ��̼��Ƶ�����Ϊ_________�ˣ������ݻ�ѧ����ʽ��ʽ���㣩
���Լ��㣺�μӷ�Ӧ��̼��Ƶ�����Ϊ_________�ˣ������ݻ�ѧ����ʽ��ʽ���㣩
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
����Ŀ����ͼ�dz��л�ѧ������ʵ��װ��ͼ��������ͼʾ�ش��������⡣
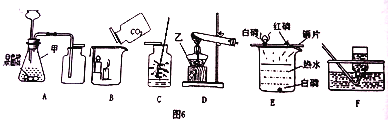
��1��д��ʵ��װ��ͼ���������ƣ���__________ ��
��2��Ҫ�ռ�һƿ�ϴ������������ѡ��_________װ�����ռ�������װ��A��ʵ����������ȡ���ռ�һ�ֳ��������װ��ͼ���������巢��װ����һ�����Դ�����_______����ȡ������Ļ�ѧ����ʽΪ_____________��
��3��װ��Bʵ��۲쵽��������____________��
��4��װ��C����˿��O2�о���ȼ�գ��������䣬�ų���������������______ɫ���塣��װ��E��ͨ���Ƚ�ͭƬ�ϰ�����ˮ�а��IJ�ͬ������ó��Ľ�����___________��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
����Ŀ����ͼ�Ǽס����������ʵ��ܽ�����ߣ���ش�
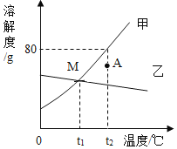
�� M���������_________________
�� t2��ʱ����40g�ļ����ʷ���ʢ��50gˮ���ձ��У�������ҺΪ____________����������������������������Һ����ʱ���ʵ���������Ϊ____________��
�� �Ӽ���Һ���ᴿ�ķ�����_________________��
�� �����ҵı�����Һ���ֻ��ǣ�ԭ����__________________________________��
������t2���ı�����Һ�������Һ�йص����ɣ�
Aˮ������ B��Һ�����ʵ����� C��Һ������ D���������� Et2��ʱ���ܽ��
�����¶Ȳ��䣬��������Һϡ�ͣ����������______________������ţ���ͬ��
����ñ�����Һ������t1�������������____________��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
����Ŀ��Сά����˾۲�ʱ���Ի��ȼ��������ƾ��������˺��棬��������ͬѧ����ɷֽ�������̽����
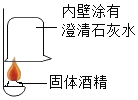
���������ϣ�
��1���ù���ƾ����þƾ����Ȼ��ƺ��������ư�һ���������Ȼ���Ƴɵġ�
��2���Ȼ��ơ��Ȼ�����Һ�������ԡ�
��������⣩
��1���ƾ����Ƿ���̼Ԫ�أ�
��2������ƾ������������Ƿ���ʣ�
���������룩
����һ���ƾ��к���̼Ԫ�ء�
�����������ƾ������������ѱ��ʡ�
��ʵ��̽����
��1����ͼ��ʾ����ʵ�飬���ֳΜ[ʯ��ˮ_____���ɵó��ƾ��к���̼Ԫ�صĽ��ۡ�
��2��ȡ��������ƾ����ձ��У���������ˮ����ܽ���ã����ֱ����а�ɫ������ȡ�������Թ��м���ϡ���ᣬ�����ܽⲢ�����ݲ�����
��ʵ����ۣ�
��1���ƾ��к���̼Ԫ�ء�
��2������ƾ������������Ѿ����ʡ�
����չ���죩Ϊ��һ��ȷ������ƾ����Ƿ����������ƣ�Сά��ͬѧ����̽����
��1������ȡ��������ƾ�����ˮ�ܽ��ȡ�ϲ���Һ���μӷ�̪��Һ����̪��Һ_____�����ǵó�����ƾ��л������������ơ�
��2��Сά��ͬѧ��Ϊ����ʵ�鲻��֤������ƾ���һ������������ʣ�࣬������_____��
��3��������ȡ�ϲ�[Һ�������������Ȼ�����Һ��������Ӧ�Ļ�ѧ����ʽΪ_____����ַ�Ӧ����ˣ�����Һ�еμ�_____������_____��������һ����Ϊ����ƾ�������������ʣ�ࡣ
����˼������ʵ��������������Ӧ_____���档
�鿴�𰸺ͽ���>>
����ʡ������Υ���Ͳ�����Ϣ�ٱ�ƽ̨ | �����к���Ϣ�ٱ�ר�� | ����թƭ�ٱ�ר�� | ����ʷ���������к���Ϣ�ٱ�ר�� | ������Ȩ�ٱ�ר��
Υ���Ͳ�����Ϣ�ٱ��绰��027-86699610 �ٱ����䣺58377363@163.com