
��9�֣��������о�ʵ������ȡ������װ��ͼ���밴Ҫ��ش��������⣮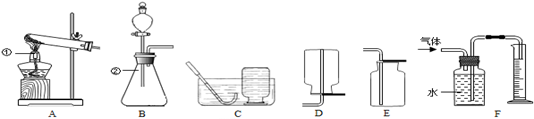
��1��д��ͼ�б�����������ƣ��� ���� ��
��2��ʵ�������������ȡ������Ӧѡ�õķ���װ���� ��������ĸ��ţ���ͬ������Ҫ�ռ�һƿ�����������Ӧѡ����ռ�װ���� ��
��3��ʵ������H2O2��Һ��MnO2���������������MnO2�� ���á�ͬѧ������B��Fװ�ã�ͨ����ˮ�����ⶨ�����������������Ӧ����������Ͳ���ռ�����ˮ��������DZ�����ֵƫ��ˮ���������ȷ��������Ҫԭ���� ��
��4������ͼ��ʾҽ�����ϴ��ſ������ռ�H2����H2
�����Ϊ ���a����b����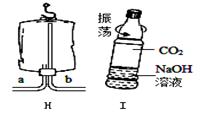
��5����ͼI��ʾ�Ŀ�Ȫˮƿ���жԱ�ʵ�飬����֤��CO2��NaOH��Һȷʵ�����˷�Ӧ��Ӧ���ĶԱ������� ��CO2��NaOH��Һ��Ӧ�Ļ�ѧ����ʽΪ ��
�Ţپƾ��� ����ƿ ��A��E �Ǵ����� ��Ӧ���ȣ��������ͣ��������������Һռ��һ�������Ҳ�У� ��b �ɽ�����������Һ���ɵ������ˮ�Ա� 2NaOH+CO2=Na2CO3+H2O
���������������1�����ݳ������������Ƽ��ɽ�𣻣�2��ʵ�������������ȡ������Ҫ����װ�ã���ѡA��Ҫ�ռ�һƿ�����������������ˮ������Ϊ�������ܶȱȿ�����Ӧѡ�������ſ���������ѡE ��ʵ������H2O2��Һ��MnO2���������������MnO2��������������ã��ų���ˮ�࣬�ռ�������࣬����ˮ�����Ϳ����Ƿ���ʹ�������ͣ�ѹǿ���� ����Ϊ�����ܶȱȿ���С��Ӧ�������ſ������ռ���������Ӧ��b��ͨ�룻����Ϊ������̼������ˮ��Ҳ��ʹ��Ȫˮƿ���Ӧ������������Һ���ɵ������ˮ�Ա� ����ƿ�ӵı��̶ȿ���ȷ�����������������̼������Ӧ��2NaOH+CO2=Na2CO3+H2O
���㣺�������������ƣ��������ȡ���ռ���ʵ�鷽������������ۣ���ѧ����ʽ����д



| �꼶 | ���пγ� | �꼶 | ���пγ� |
| ��һ | ��һ��ѿγ��Ƽ��� | ��һ | ��һ��ѿγ��Ƽ��� |
| �߶� | �߶���ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
| ���� | ������ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
��Ŀ�����л�ѧ ��Դ�� ���ͣ������
ij��ȤС����������������װ�ý���ʵ�飬��ش�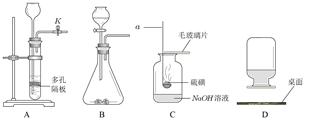
�� Aװ����Ϊ����װ�ÿ�����ȡ�������� ��1�� ���رջ���K���۲쵽�������� ��2�� ����Ӧֹͣ���ٲ������ݡ�
�� �ù�������Ͷ���������ȡ������Ϊ�õ�ƽ�ȵ���������ѡ�õķ���װ��Ϊ ��3�� ������װ�ñ�ţ�
�� ��Cװ���������������ȼ��ʵ�飬��������a�������� ��4�� ��ȼ�ս�����ȡ������a�������ò���Ƭ��סƿ�ڲ���������ƿ��ת��D��ʾ������Ƭ������䣬ԭ����
��5�� ���û�ѧ����ʽ��ʾ����
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ������
ʵ������ȡ���峣���õ�����װ�ã����ݸ�����װ�ûش��������⣺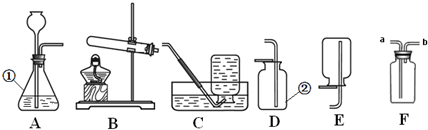
��1��д��������������ƣ��� ��
��2������ʵ��ѡ��װ��A����ȡ��������д���÷�Ӧ�Ļ�ѧ����ʽ ����Cװ���ռ��������ж��������ռ����������� ��
��3��ijͬѧ������װ��ͼ��ѡ���ʵ�װ�óɹ����Ʊ����ռ��˶�����̼��ѡ�õ�װ����
������ĸ����Ϊ�˽�һ����֤�����������Ƕ�����̼����ͬѧ������ͨ��ͼFװ���У���Fװ����Ӧ������Լ�Ϊ ���ѧʽ����
��4����Ҫ��ȡ��������Fװ���ռ�������Ӧ�� ���a����b�������ܿ�ͨ�롣
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ������
(7��)���������ʵ��װ��ͼ�ش����⣺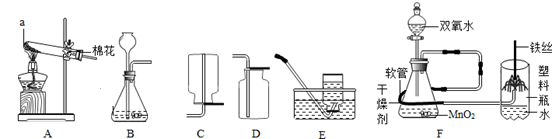
�� д������a�����ƣ�a ��
�� ��Aװ����ȡO2�Ļ�ѧ����ʽΪ___________________________���ռ��ϴ�������װ��Ϊ_________����װ�ñ�ţ���
�� ��B��Cװ����Ͽ�����ȡ��һ��������______����ѧ����ʽΪ_________________��
�� ��ͼF�ǡ���˿��������ȼ�ա�ʵ��ĸĽ�װ�á�ʵ��ʱ����Һ©��������ͨ���������Լ10�룬��ȼ��˿�¶˻��ˣ���������ƿ�ڣ����������ܿ����Ϸ����۲쵽�������ǣ���˿����ȼ�գ� ���Ľ�����ŵ��� ������ţ���
������������ǰ�Ʊ����ռ�������������
������ƿ���漯��ƿ����ֹ����ƿը�ѣ�����ȫ
��װ�ü���������ȡ�������������֤��һ�壬ʵ����Ż�
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ������
��10�֣���������ͼ��ʾװ�ã������ѧ֪ʶ�ش��������⡣
��1��д��ͼ�������ٵ����� ��
��2��ʵ������Aװ����ȡ������д����Ӧ�Ļ�ѧ����ʽ ��
��3��ʵ������ȡ������̼Ӧѡ�õķ���װ���� ��Ҫд�����Ļ�ѧ����ʽ�� ��
��4��ʵ������B��E��Cװ����ȡ���ռ��������������E��Ӧʢ�ŵ��Լ��� ��
��5��ʵ������Dװ���ռ��������� ʱ��ʼ�ռ���
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ������
����ʵ��װ���dz��л�ѧʵ���ҳ���װ�ã���A�⣩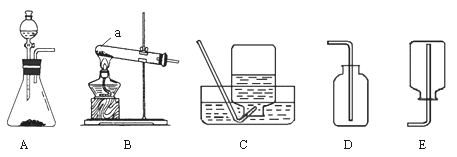
�������ʵ�����������ʶ���ش��������⣺
��1��ѡ��C��G�� ��ϳ�װ�ÿ���ȡ�����������
��2��ѡB����ȡ����ķ���װ�ã�Ӧ�����������
��3������Gװ���ռ�������̼���ӷ���װ�ò����Ķ�����̼Ӧ��G�� ������ҡ�����ͨ�룬�������������̼�Ƿ����ķ�����
��4��Cװ�������Ľ��������ľ̿��ԭ����ͭʵ�飬�Ľ�������
�������ʵ��ԭ����������о������Խ���������⣺
|
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ������
������ͼ��ʾ��װ��ͼ���ش��������⣺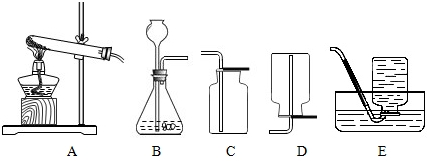
��1����A��C��װ���Ʊ�һ�����壬��д���Ʊ�������Ļ�ѧ����ʽ����˵����μ�������壿
��2��ͨ���ȽϷ�����ȡ������̼�������������ķ���װ�ö�����ѡ��Bװ�ã��Դӷ�Ӧ���״̬����Ӧ�����ȷ����ܽ�����Bװ��Ҫ���һ����������Щ��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ������
��7�֣�������ʾΪʵ�����г��������Ʊ�������ռ�������ʵ��IJ�����������װʵ��װ��ʱ�����ظ�ѡ�����������Ը�����ĿҪ�ش��������⣺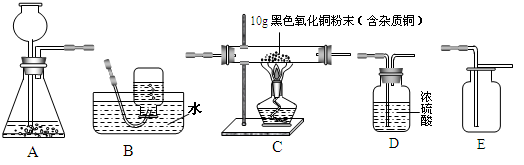
��1�����Թ���������ҺΪԭ�ϣ�������������������ʵ�������Ʊ����ռ������������
����ѡ����������˳��Ϊ_______________����д���������ĸ����
����������ʱ������A�з�����Ӧ�Ļ�ѧ����ʽΪ ��
��2������п��ϡ���ᷴӦ��ȡ�������������ⶨij����������ͭ��Ʒ�Ĵ��ȣ�����Ϊ��������ͭ������ѡ����������˳��Ϊ��A��D1��C��D2��D3������֪��CuO+H2 Cu+H2O��D1��D2��D3Ϊ3��Ũ����ϴ��ƿ��
Cu+H2O��D1��D2��D3Ϊ3��Ũ����ϴ��ƿ��
������D1��������_____________________________________________��
������A�з�����Ӧ�Ļ�ѧ����ʽ�� ��
�۷�Ӧ��ɺ�װ��D2��Ũ�������������1.8g��������ͭ��Ʒ�Ĵ���Ϊ ����������D1������������ͭ��Ʒ�Ĵ��Ƚ���_____________���ƫ����ƫС������һ�¡�����
����ͨ�� �ķ�������ʹ������D1�����������ͭ��Ʒ�Ĵ���Ҳ�����һ�¡�
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ������
��8�֣�������ͼ�ش����⡣
С�ں�С���������Ӻõ�E��F��Gװ����ʵ�顣������E�����ӵ�ҩƷ��ͬ��G������ҩƷ��ͬ��С��ʵ��ʱ����F�е�����Ϩ��G�е���Һ����ǣ���E�з�����Ӧ�Ļ�ѧ����ʽ�� ��С����ʵ��ʱ����F�е�����ȼ�յĸ�����G�е���ҺҲ����ǣ���E�з�����Ӧ�Ļ�ѧ����ʽ�� ��
��3��С�����һ��̽��G�а�ɫ������ijɷ֡�����G�еİ�ɫ�������Һ���з�������IJ��������� ����һ���IJ��������ɫ�������м��� ��д�Լ����ƣ���
�鿴�𰸺ͽ���>>
����ʡ������Υ���Ͳ�����Ϣ�ٱ�ƽ̨ | �����к���Ϣ�ٱ�ר�� | ����թƭ�ٱ�ר�� | ����ʷ���������к���Ϣ�ٱ�ר�� | ������Ȩ�ٱ�ר��
Υ���Ͳ�����Ϣ�ٱ��绰��027-86699610 �ٱ����䣺58377363@163.com