
ŌŚŅ»ÉÕ±ÖŠŹ¢ÓŠ22.3æĖĢ¼ĖįÄĘŗĶĀČ»ÆÄĘ×é³ÉµÄ¹ĢĢå»ģŗĻĪļ£¬ĻņĘäÖŠÖš½„µĪ¼ÓČÜÖŹÖŹĮæ·ÖŹżĪŖ10%µÄĻ”ŃĪĖį£ØNa2CO3+2HCl=2NaCl+H2O+CO2”ü£©£¬·Å³öĘųĢåµÄ×ÜÖŹĮæÓėĖłµĪČėĻ”ŃĪĖįµÄÖŹĮæ¹ŲĻµĒśĻßČēĶ¼ĖłŹ¾”£Ēėøł¾ŻĢāŅā»Ų“šĪŹĢā£ŗ
£Ø1£©µ±µĪ¼ÓĮĖ73æĖĻ”ŃĪĖįŹ±£¬·Å³öĘųĢåµÄ×ÜÖŹĮæĪŖ ”ų æĖ”£
£Ø2£©µ±µĪ¼ÓĻ”ŃĪĖįÖĮĶ¼ÖŠµÄBµćŹ±£¬ÉÕ±ÖŠČÜŅŗĄļµÄČÜÖŹŹĒ£ØŠ“»ÆѧŹ½£©£ŗ ”ų ”£
£Ø3£©µ±µĪ¼ÓĮĖ73æĖĻ”ŃĪĖįŹ±£Ø¼“AµćŹ±£©£¬ÉÕ±ÖŠĪŖ²»±„ŗĶČÜŅŗ£¬ŹŌĶعż¼ĘĖćĒó³ö“ĖŹ±ĖłµĆČÜŅŗµÄČÜÖŹµÄÖŹĮæ·ÖŹż”££Ø×¼Č·µ½0.1%£©

| Äź¼¶ | øßÖŠæĪ³Ģ | Äź¼¶ | ³õÖŠæĪ³Ģ |
| øßŅ» | øßŅ»Ćā·ŃæĪ³ĢĶĘ¼ö£” | ³õŅ» | ³õŅ»Ćā·ŃæĪ³ĢĶĘ¼ö£” |
| ø߶ž | ø߶žĆā·ŃæĪ³ĢĶĘ¼ö£” | ³õ¶ž | ³õ¶žĆā·ŃæĪ³ĢĶĘ¼ö£” |
| øßČż | øßČżĆā·ŃæĪ³ĢĶĘ¼ö£” | ³õČż | ³õČżĆā·ŃæĪ³ĢĶĘ¼ö£” |
æĘÄæ£ŗ³õÖŠ»Æѧ Ą“Ō“£ŗ ĢāŠĶ£ŗ
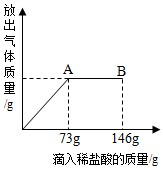 ŌŚŅ»ÉÕ±ÖŠŹ¢ÓŠ22.3gNa2CO3ŗĶNaCl×é³ÉµÄ¹ĢĢå»ģŗĻĪļ£¬ĻņĘäÖŠÖš½„µĪ¼ÓČÜÖŹÖŹĮæ·ÖŹżĪŖ10%µÄĻ”ŃĪĖį£¬·Å³öĘųĢåµÄ×ÜÖŹĮæÓėĖłµĪČėĻ”ŃĪĖįµÄÖŹĮæ¹ŲĻµĒśĻßČēĶ¼ĖłŹ¾£ŗĒėøł¾ŻĢāŅā»Ų“šĪŹĢā£ŗ
ŌŚŅ»ÉÕ±ÖŠŹ¢ÓŠ22.3gNa2CO3ŗĶNaCl×é³ÉµÄ¹ĢĢå»ģŗĻĪļ£¬ĻņĘäÖŠÖš½„µĪ¼ÓČÜÖŹÖŹĮæ·ÖŹżĪŖ10%µÄĻ”ŃĪĖį£¬·Å³öĘųĢåµÄ×ÜÖŹĮæÓėĖłµĪČėĻ”ŃĪĖįµÄÖŹĮæ¹ŲĻµĒśĻßČēĶ¼ĖłŹ¾£ŗĒėøł¾ŻĢāŅā»Ų“šĪŹĢā£ŗ²éæ““š°øŗĶ½āĪö>>
æĘÄæ£ŗ³õÖŠ»Æѧ Ą“Ō“£ŗ ĢāŠĶ£ŗŌĶĮĄķ½ā
²éæ““š°øŗĶ½āĪö>>
æĘÄæ£ŗ³õÖŠ»Æѧ Ą“Ō“£ŗ ĢāŠĶ£ŗ
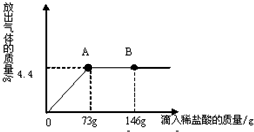
 ŌŚŅ»ÉÕ±ÖŠŹ¢ÓŠ22.3g Na2CO3ŗĶNaCl×é³ÉµÄ¹ĢĢå»ģŗĻĪļ£¬¼Ó143.1gĖ®Čܽā£¬ÖĘ³ÉČÜŅŗ£®
ŌŚŅ»ÉÕ±ÖŠŹ¢ÓŠ22.3g Na2CO3ŗĶNaCl×é³ÉµÄ¹ĢĢå»ģŗĻĪļ£¬¼Ó143.1gĖ®Čܽā£¬ÖĘ³ÉČÜŅŗ£®²éæ““š°øŗĶ½āĪö>>
æĘÄæ£ŗ³õÖŠ»Æѧ Ą“Ō“£ŗ ĢāŠĶ£ŗ
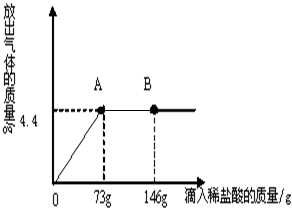
 £Ø2013?Ą„¶¼ĀŲĒųŅ»Ä££©ŌŚŅ»ÉÕ±ÖŠŹ¢ÓŠ22.3Na2CO3ŗĶNaCl×é³ÉµÄ¹ĢĢå»ģŗĻĪļ£¬¼ÓČė109.1g Ė®Ź¹ĘäĶźČ«Čܽā£¬Åä³ÉČÜŅŗ£®ĻņĘäÖŠÖš½„µĪ¼ÓČÜÖŹÖŹĮæ·ÖŹżĪŖ10%µÄĻ”ŃĪĖį£¬·Å³öĘųĢåµÄ×ÜÖŹĮæÓėĖłµĪČėĻ”ŃĪĖįµÄÖŹĮæ¹ŲĻµĒśĻßČēĶ¼ĖłŹ¾£®Ēėøł¾ŻĢāŅā»Ų“šĪŹĢā£ŗ
£Ø2013?Ą„¶¼ĀŲĒųŅ»Ä££©ŌŚŅ»ÉÕ±ÖŠŹ¢ÓŠ22.3Na2CO3ŗĶNaCl×é³ÉµÄ¹ĢĢå»ģŗĻĪļ£¬¼ÓČė109.1g Ė®Ź¹ĘäĶźČ«Čܽā£¬Åä³ÉČÜŅŗ£®ĻņĘäÖŠÖš½„µĪ¼ÓČÜÖŹÖŹĮæ·ÖŹżĪŖ10%µÄĻ”ŃĪĖį£¬·Å³öĘųĢåµÄ×ÜÖŹĮæÓėĖłµĪČėĻ”ŃĪĖįµÄÖŹĮæ¹ŲĻµĒśĻßČēĶ¼ĖłŹ¾£®Ēėøł¾ŻĢāŅā»Ų“šĪŹĢā£ŗ²éæ““š°øŗĶ½āĪö>>
æĘÄæ£ŗ³õÖŠ»Æѧ Ą“Ō“£ŗ ĢāŠĶ£ŗŌĶĮĄķ½ā
 Š”øÕŌŚ»ÆѧŹµŃéŹŅ·¢ĻÖ£¬Ź¢·ÅNaOHČÜŅŗµÄŹŌ¼ĮĘæĘææŚŗĶĻšĘ¤ČūÉĻ³öĻÖĮĖ°×É«·ŪÄ©£®Š”øÕ½ŠĄ“Š”¾üŗĶŠ”ŗģ£¬¹²Ķ¬Ģ½¾æÕāÖÖ°×É«·ŪÄ©µÄ³É·Ö£®
Š”øÕŌŚ»ÆѧŹµŃéŹŅ·¢ĻÖ£¬Ź¢·ÅNaOHČÜŅŗµÄŹŌ¼ĮĘæĘææŚŗĶĻšĘ¤ČūÉĻ³öĻÖĮĖ°×É«·ŪÄ©£®Š”øÕ½ŠĄ“Š”¾üŗĶŠ”ŗģ£¬¹²Ķ¬Ģ½¾æÕāÖÖ°×É«·ŪÄ©µÄ³É·Ö£®²éæ““š°øŗĶ½āĪö>>
°Ł¶ČÖĀŠÅ - Į·Ļ°²įĮŠ±ķ - ŹŌĢāĮŠ±ķ
ŗž±±Ź”»„ĮŖĶųĪ„·ØŗĶ²»Į¼ŠÅĻ¢¾Ł±ØĘ½ĢØ | ĶųÉĻÓŠŗ¦ŠÅĻ¢¾Ł±Ø×ØĒų | µēŠÅÕ©Ę¾Ł±Ø×ØĒų | É꥜Ź·ŠéĪŽÖ÷ŅåÓŠŗ¦ŠÅĻ¢¾Ł±Ø×ØĒų | ÉęĘóĒÖČؾŁ±Ø×ØĒų
Ī„·ØŗĶ²»Į¼ŠÅĻ¢¾Ł±Øµē»°£ŗ027-86699610 ¾Ł±ØÓŹĻä£ŗ58377363@163.com