
 ĪŖ²ā¶Øij“æ¼ī£ØNa2CO3£©ŃłĘ·ÖŠ£Øŗ¬ÓŠÉŁĮæµÄĀČ»ÆÄĘŌÓÖŹ£©Ģ¼ĖįÄʵÄÖŹĮæ·ÖŹż£¬
ĪŖ²ā¶Øij“æ¼ī£ØNa2CO3£©ŃłĘ·ÖŠ£Øŗ¬ÓŠÉŁĮæµÄĀČ»ÆÄĘŌÓÖŹ£©Ģ¼ĖįÄʵÄÖŹĮæ·ÖŹż£¬| Ģ¼ĖįÄʵÄÖŹĮæ |
| ×ÜÖŹĮæ |
| 106 |
| x |
| 73 |
| y |
| 44 |
| 2.2g |
| 5.3g |
| 6g |
| 3.65g |
| 36.5g |


 ŌĶĮæģ³µĻµĮŠ“š°ø
ŌĶĮæģ³µĻµĮŠ“š°ø
| Äź¼¶ | øßÖŠæĪ³Ģ | Äź¼¶ | ³õÖŠæĪ³Ģ |
| øßŅ» | øßŅ»Ćā·ŃæĪ³ĢĶĘ¼ö£” | ³õŅ» | ³õŅ»Ćā·ŃæĪ³ĢĶĘ¼ö£” |
| ø߶ž | ø߶žĆā·ŃæĪ³ĢĶĘ¼ö£” | ³õ¶ž | ³õ¶žĆā·ŃæĪ³ĢĶĘ¼ö£” |
| øßČż | øßČżĆā·ŃæĪ³ĢĶĘ¼ö£” | ³õČż | ³õČżĆā·ŃæĪ³ĢĶĘ¼ö£” |
æĘÄæ£ŗ³õÖŠ»Æѧ Ą“Ō“£ŗ ĢāŠĶ£ŗ
 ÓĆ”°ŗīŹĻĮŖŗĻÖĘ¼ī·Ø”±ÖʵƵēæ¼ī³£ŗ¬ÓŠÉŁĮæµÄĀČ»ÆÄĘ£®ĪŖ²ā¶Øij“æ¼īѳʷ֊Ģ¼ĖįÄʵÄŗ¬Į棬Š”Ć÷³ĘČ”øĆ“æ¼īѳʷ10gČܽāÓŚĖ®ÖŠ£¬ŌŁµĪ¼ÓĀČ»ÆøĘČÜŅŗ£¬²śÉś³ĮµķµÄÖŹĮæČēĶ¼ĖłŹ¾£¬Ēó£ŗøĆ“æ¼īѳʷ֊Ģ¼ĖįÄʵÄÖŹĮæ·ÖŹż£®
ÓĆ”°ŗīŹĻĮŖŗĻÖĘ¼ī·Ø”±ÖʵƵēæ¼ī³£ŗ¬ÓŠÉŁĮæµÄĀČ»ÆÄĘ£®ĪŖ²ā¶Øij“æ¼īѳʷ֊Ģ¼ĖįÄʵÄŗ¬Į棬Š”Ć÷³ĘČ”øĆ“æ¼īѳʷ10gČܽāÓŚĖ®ÖŠ£¬ŌŁµĪ¼ÓĀČ»ÆøĘČÜŅŗ£¬²śÉś³ĮµķµÄÖŹĮæČēĶ¼ĖłŹ¾£¬Ēó£ŗøĆ“æ¼īѳʷ֊Ģ¼ĖįÄʵÄÖŹĮæ·ÖŹż£®²éæ““š°øŗĶ½āĪö>>
æĘÄæ£ŗ³õÖŠ»Æѧ Ą“Ō“£ŗ ĢāŠĶ£ŗ
 ĪŖ²ā¶Øij“æ¼ī£ØNa2CO3£©ŃłĘ·ÖŠ£Øŗ¬ÓŠÉŁĮæµÄĀČ»ÆÄĘŌÓÖŹ£©Ģ¼ĖįÄʵÄÖŹĮæ·ÖŹż£¬ĻÖ³ĘČ”6gŹŌŃł·ÅŌŚÉÕ±ÖŠ²¢µĪČėĻ”ŃĪĖį£¬µ±Ļ”ŃĪĖįµĪ¼ÓÖĮ36.5gŹ±£¬ÉÕ±ÄŚČÜŅŗµÄ×ÜÖŹĮæĪŖ40.3g£Ø²śÉśµÄĘųĢåČ«²æŅŻ³ö£©£®²śÉśĘųĢåµÄÖŹĮæÓėµĪČėĻ”ŃĪĖįµÄÖŹĮæ¹ŲĻµČēĶ¼ĖłŹ¾£®
ĪŖ²ā¶Øij“æ¼ī£ØNa2CO3£©ŃłĘ·ÖŠ£Øŗ¬ÓŠÉŁĮæµÄĀČ»ÆÄĘŌÓÖŹ£©Ģ¼ĖįÄʵÄÖŹĮæ·ÖŹż£¬ĻÖ³ĘČ”6gŹŌŃł·ÅŌŚÉÕ±ÖŠ²¢µĪČėĻ”ŃĪĖį£¬µ±Ļ”ŃĪĖįµĪ¼ÓÖĮ36.5gŹ±£¬ÉÕ±ÄŚČÜŅŗµÄ×ÜÖŹĮæĪŖ40.3g£Ø²śÉśµÄĘųĢåČ«²æŅŻ³ö£©£®²śÉśĘųĢåµÄÖŹĮæÓėµĪČėĻ”ŃĪĖįµÄÖŹĮæ¹ŲĻµČēĶ¼ĖłŹ¾£®²éæ““š°øŗĶ½āĪö>>
æĘÄæ£ŗ³õÖŠ»Æѧ Ą“Ō“£ŗ ĢāŠĶ£ŗ
²éæ““š°øŗĶ½āĪö>>
æĘÄæ£ŗ³õÖŠ»Æѧ Ą“Ō“£ŗ ĢāŠĶ£ŗ

²éæ““š°øŗĶ½āĪö>>
æĘÄæ£ŗ³õÖŠ»Æѧ Ą“Ō“£ŗ ĢāŠĶ£ŗ
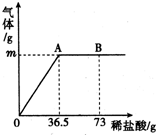 ĪŖ²ā¶Øij“æ¼īѳʷ֊£Øŗ¬ÓŠÉŁĮæµÄĀČ»ÆÄĘŌÓÖŹ£©Ģ¼ĖįÄʵÄÖŹĮæ·ÖŹż£¬ĻÖ³ĘČ”6gŹŌŃł·ÅŌŚÉÕ±ÖŠ²¢µĪČėĻ”ŃĪĖį£¬µ±Ļ”ŃĪĖįµĪ¼ÓÖĮ36.5gŹ±£¬ÉÕ±ÄŚČÜŅŗµÄ×ÜÖŹĮæĪŖ40.3g£Ø²śÉśµÄĘųĢåČ«²æŅŻ³ö£©£®²śÉśĘųĢåµÄÖŹĮæÓėµĪČėĻ”ŃĪĖįµÄÖŹĮæ¹ŲĻµČēĶ¼ĖłŹ¾£¬ŹŌ¼ĘĖć£ŗ
ĪŖ²ā¶Øij“æ¼īѳʷ֊£Øŗ¬ÓŠÉŁĮæµÄĀČ»ÆÄĘŌÓÖŹ£©Ģ¼ĖįÄʵÄÖŹĮæ·ÖŹż£¬ĻÖ³ĘČ”6gŹŌŃł·ÅŌŚÉÕ±ÖŠ²¢µĪČėĻ”ŃĪĖį£¬µ±Ļ”ŃĪĖįµĪ¼ÓÖĮ36.5gŹ±£¬ÉÕ±ÄŚČÜŅŗµÄ×ÜÖŹĮæĪŖ40.3g£Ø²śÉśµÄĘųĢåČ«²æŅŻ³ö£©£®²śÉśĘųĢåµÄÖŹĮæÓėµĪČėĻ”ŃĪĖįµÄÖŹĮæ¹ŲĻµČēĶ¼ĖłŹ¾£¬ŹŌ¼ĘĖć£ŗ²éæ““š°øŗĶ½āĪö>>
°Ł¶ČÖĀŠÅ - Į·Ļ°²įĮŠ±ķ - ŹŌĢāĮŠ±ķ
ŗž±±Ź”»„ĮŖĶųĪ„·ØŗĶ²»Į¼ŠÅĻ¢¾Ł±ØĘ½ĢØ | ĶųÉĻÓŠŗ¦ŠÅĻ¢¾Ł±Ø×ØĒų | µēŠÅÕ©Ę¾Ł±Ø×ØĒų | É꥜Ź·ŠéĪŽÖ÷ŅåÓŠŗ¦ŠÅĻ¢¾Ł±Ø×ØĒų | ÉęĘóĒÖČؾŁ±Ø×ØĒų
Ī„·ØŗĶ²»Į¼ŠÅĻ¢¾Ł±Øµē»°£ŗ027-86699610 ¾Ł±ØÓŹĻä£ŗ58377363@163.com