
ij��ȤС��ͬѧ�ڸ�ϰ������̼�Ļ�ѧ����ʱ������ͼ5��ʾ��ʵ�飬����ͼʾ�ش��������⡣
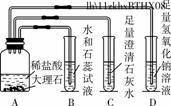 ͼ5
ͼ5
(1)װ��B��ʯ����Һ��������________________________________________________________________________��
װ��D�з�Ӧ�Ļ�ѧ����ʽ��________________________________________________________________________��
(2)ʵ�������ͬѧ�ǰ��������������ʵ���ͬһ����Һ���У����ַ�Һ���Dz�����ɫ��˵����Һ��һ��û��(д��ѧʽ����ͬ)________��
(3)Ϊ�˿�ѧ����ʵ�������ķ�Һ��ͬѧ���Ƚ���Һ���ˣ�����õ�����Һ����μ���ϡ���ᣬ������ʼʱ����������һ��������ݲ�������Һ����ɫ��Ϊ��ɫ������Һ�е����ʳ�ָʾ���һ������________________________________________________________________________��
һ��û��______________��
 ��������һ���þ�ϵ�д�
��������һ���þ�ϵ�д� Сѧ��10����Ӧ����ϵ�д�
Сѧ��10����Ӧ����ϵ�д�
| �꼶 | ���пγ� | �꼶 | ���пγ� |
| ��һ | ��һ��ѿγ��Ƽ��� | ��һ | ��һ��ѿγ��Ƽ��� |
| �߶� | �߶���ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
| ���� | ������ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
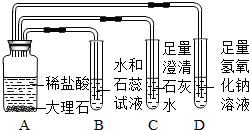
ij��ȤС��ͬѧ�ڸ�ϰ������̼�Ļ�ѧ����ʱ������ͼ5��ʾ��ʵ�飬����ͼʾ�ش��������⡣
(1)װ��B��ʯ����Һ��������___________________________________��װ��D�з�Ӧ�Ļ�ѧ����ʽ��_______________________________________________��
(2)ʵ�������ͬѧ�ǰ��������������ʵ���ͬһ����Һ���У����ַ�Һ���Dz�����ɫ��˵����Һ��һ��û��(д��ѧʽ����ͬ)________��
(3)Ϊ�˿�ѧ����ʵ�������ķ�Һ��ͬѧ���Ƚ���Һ���ˣ�����õ�����Һ����μ���ϡ���ᣬ������ʼʱ����������һ��������ݲ�������Һ����ɫ��Ϊ��ɫ������Һ�е����ʳ�ָʾ���һ������______________________________��һ��û��______________��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
�鿴�𰸺ͽ���>>
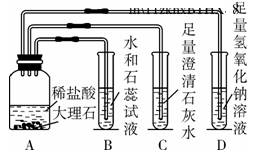
��Ŀ�����л�ѧ ��Դ��2011����б�ҵ��ѧ���ԣ����ɹŰ�ͷ������ѧ ���ͣ������
ij��ȤС��ͬѧ�ڸ�ϰ������̼�Ļ�ѧ����ʱ������ͼ5��ʾ��ʵ�飬����ͼʾ�ش��������⡣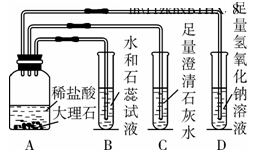
(1)װ��B��ʯ����Һ��������___________________________________��װ��D�з�Ӧ�Ļ�ѧ����ʽ��_______________________________________________��
(2)ʵ�������ͬѧ�ǰ��������������ʵ���ͬһ����Һ���У����ַ�Һ���Dz�����ɫ��˵����Һ��һ��û��(д��ѧʽ����ͬ)________��
(3)Ϊ�˿�ѧ����ʵ�������ķ�Һ��ͬѧ���Ƚ���Һ���ˣ�����õ�����Һ����μ���ϡ���ᣬ������ʼʱ����������һ��������ݲ�������Һ����ɫ��Ϊ��ɫ������Һ�е����ʳ�ָʾ���һ������______________________________��һ��û��______________��
�鿴�𰸺ͽ���>>
����ʡ������Υ���Ͳ�����Ϣ�ٱ�ƽ̨ | �����к���Ϣ�ٱ�ר�� | ����թƭ�ٱ�ר�� | ����ʷ���������к���Ϣ�ٱ�ר�� | ������Ȩ�ٱ�ר��
Υ���Ͳ�����Ϣ�ٱ��绰��027-86699610 �ٱ����䣺58377363@163.com