
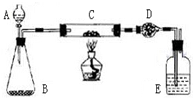
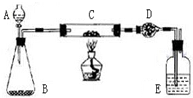
 ij����С�����̿�ۣ�������������ͭ�Ļ���������ͼװ�ã��Ի�õ�ͭ�ۣ���̿����Ʒ����ʵ�飮ͼ������̨��װ������ȥ��������������������ʵ�鱨�森
ij����С�����̿�ۣ�������������ͭ�Ļ���������ͼװ�ã��Ի�õ�ͭ�ۣ���̿����Ʒ����ʵ�飮ͼ������̨��װ������ȥ��������������������ʵ�鱨�森| ʵ����� | ʵ������ | �йػ�ѧ����ʽ |
| ��C�м�����Ʒ�걾W�ˣ�D��װ��ҩƷ����Ϊm1�ˣ����Ӻ�������������� | \ | \ |
| ��A�Ļ����������μ�Һ�� | ________ | ________ |
| ��C���м��ȣ���C��ҩƷ��ַ�Ӧ�ر�A�Ļ�����ֹͣ���� | ________ | ________ |
| ��ȴ����D������Ϊm2�� | \ | \ |
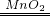 2H2O+O2�� C�еĺ��ɫ��ĩ��ɺ�ɫ 2Cu+O2
2H2O+O2�� C�еĺ��ɫ��ĩ��ɺ�ɫ 2Cu+O2 2CuO
2CuO CO2
CO2 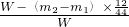
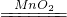 2H2O+O2��
2H2O+O2�� 2CuO��C+O2
2CuO��C+O2 CO2
CO2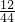 ������Ʒ��ͭ����������Ϊ
������Ʒ��ͭ����������Ϊ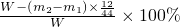
| B��E�������ݲ��� | 2H2O2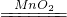 2H2O+O2�� 2H2O+O2�� |
| C�еĺ��ɫ��ĩ��ɺ�ɫ | 2Cu+O2 2CuO 2CuOC+O2  CO2 CO2 |
 ��100%
��100%

 �Ķ��쳵ϵ�д�
�Ķ��쳵ϵ�д�
| �꼶 | ���пγ� | �꼶 | ���пγ� |
| ��һ | ��һ��ѿγ��Ƽ��� | ��һ | ��һ��ѿγ��Ƽ��� |
| �߶� | �߶���ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
| ���� | ������ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
��Ŀ�����л�ѧ ��Դ�� ���ͣ��Ķ�����

| ʵ�鲽�� | ʵ������ | �йػ�ѧ����ʽ |
| ��C�м�����Ʒ��ĩW g��D��װ��ҩƷ����Ϊm1 g�����Ӻ������� | ||
| ��A�������������������μ���Һ�� | ||
| ��C���м��ȣ���C��ҩƷ��ַ�Ӧ�� ��A�Ļ�����ֹͣ���ȣ� | ||
| ��ȴ����D������Ϊm2 g�� |
W-(m2-m1)��
| ||
| W |
W-(m2-m1)��
| ||
| W |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
 ij����С�����̿�ۣ�������������ͭ�Ļ���������ͼװ�ã��Ի�õ�ͭ�ۣ���̿����Ʒ����ʵ�飮ͼ������̨��װ������ȥ��������������������ʵ�鱨�森
ij����С�����̿�ۣ�������������ͭ�Ļ���������ͼװ�ã��Ի�õ�ͭ�ۣ���̿����Ʒ����ʵ�飮ͼ������̨��װ������ȥ��������������������ʵ�鱨�森| ʵ����� | ʵ������ | �йػ�ѧ����ʽ | ||||||||||||||||
| ��C�м�����Ʒ�걾W�ˣ�D��װ��ҩƷ����Ϊm1�ˣ����Ӻ�������������� | \ | \ | ||||||||||||||||
| ��A�Ļ����������μ�Һ�� | B��E�������ݲ��� B��E�������ݲ��� |
2H2O2
2H2O2
| ||||||||||||||||
| ��C���м��ȣ���C��ҩƷ��ַ�Ӧ�ر�A�Ļ�����ֹͣ���� | C�еĺ��ɫ��ĩ��ɺ�ɫ C�еĺ��ɫ��ĩ��ɺ�ɫ |
2Cu+O2
C+O2
2Cu+O2
C+O2
| ||||||||||||||||
| ��ȴ����D������Ϊm2�� | \ | \ |
W-(m2-m1)��
| ||
| W |
W-(m2-m1)��
| ||
| W |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
ij����С�����̿��(����)������ͭ�Ļ���������ͼ��ʾװ�ã��Ի�õ�ͭ��(��̿)��Ʒ����ʵ�顣ͼ������̨��װ������ȥ��������������������ʵ�鱨�档
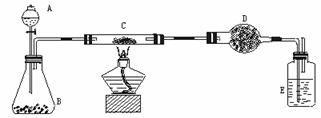
(һ)ʵ��Ŀ�ģ� ��
(��)ʵ����Ʒ��
��������ƽ����Һ©������ƿ��Ӳ�ʲ����ܡ�����ܡ��ƾ��ơ�ϴ��ƿ�ȡ�
ҩƷ�����ɫ(��̿)��Ʒ������������Һ���������̡���ʯ�ң������������ƺ������ƵĻ�����Ũ����ȡ�
(��)ʵ�����ݣ�
| ʵ�鲽�� | ʵ������ | �йػ�ѧ����ʽ |
| ��C�м�����Ʒ��ĩW g��D��װ��ҩƷ����Ϊm1 g�����Ӻ������� | ||
| ��A�������������������μ���Һ�� | ||
| ��C���м��ȡ���C��ҩƷ��ַ�Ӧ�ر�A�Ļ�����ֹͣ���ȡ� | ||
| ��ȴ����D������Ϊm2 g�� |
(��)���㣺��Ʒ��ͭ������������ (�ú�����m1��m2�Ĵ���ʽ��ʾ)
(��)��������ۣ�
ʵ����ɺ���ʦ����˵��������ʵ����ƣ���ʹC�з�Ӧ��ȫ��D��������ȫ��Ҳ����ó���ȷ�Ľ���������ۣ���ͬѧ�����B��C֮�����һ��װ�á��ٴ�ʵ��õ��˽���ȷ�Ľ������ô��ԭ��ʵ������õ�ͭ����������ƫС��ԭ������� ����B��C֮������װ�ÿ����� ������ʢ�ŵ�ҩƷ�� ��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ��2011��ڶ�ʮһ�조��ԭ����ȫ������ѧ����ѧ���ʺ�ʵ��������������ɽ�����������Ծ��������棩 ���ͣ������
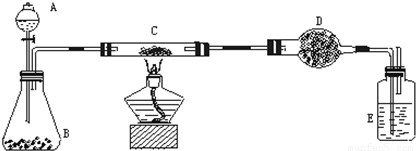
| ʵ�鲽�� | ʵ������ | �йػ�ѧ����ʽ |
| ��C�м�����Ʒ��ĩW g��D��װ��ҩƷ����Ϊm1 g�����Ӻ������� | ||
| ��A�������������������μ���Һ�� | ||
| ��C���м��ȣ���C��ҩƷ��ַ�Ӧ�� ��A�Ļ�����ֹͣ���ȣ� | ||
| ��ȴ����D������Ϊm2 g�� |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ��2004�����ʡ�Ƹ����п���ѧģ���Ծ��������棩 ���ͣ������
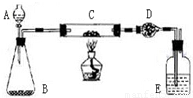 ij����С�����̿�ۣ�������������ͭ�Ļ���������ͼװ�ã��Ի�õ�ͭ�ۣ���̿����Ʒ����ʵ�飮ͼ������̨��װ������ȥ��������������������ʵ�鱨�森
ij����С�����̿�ۣ�������������ͭ�Ļ���������ͼװ�ã��Ի�õ�ͭ�ۣ���̿����Ʒ����ʵ�飮ͼ������̨��װ������ȥ��������������������ʵ�鱨�森| ʵ����� | ʵ������ | �йػ�ѧ����ʽ |
| ��C�м�����Ʒ�걾W�ˣ�D��װ��ҩƷ����Ϊm1�ˣ����Ӻ�������������� | \ | \ |
| ��A�Ļ����������μ�Һ�� | ______ | ______ |
�鿴�𰸺ͽ���>>
����ʡ������Υ���Ͳ�����Ϣ�ٱ�ƽ̨ | �����к���Ϣ�ٱ�ר�� | ����թƭ�ٱ�ר�� | ����ʷ���������к���Ϣ�ٱ�ר�� | ������Ȩ�ٱ�ר��
Υ���Ͳ�����Ϣ�ٱ��绰��027-86699610 �ٱ����䣺58377363@163.com