


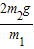 �� ƫ�ͣ�
�� ƫ�ͣ�

 ������ÿ�ʱ�Ż���ҵϵ�д�
������ÿ�ʱ�Ż���ҵϵ�д�
| �꼶 | ���пγ� | �꼶 | ���пγ� |
| ��һ | ��һ��ѿγ��Ƽ��� | ��һ | ��һ��ѿγ��Ƽ��� |
| �߶� | �߶���ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
| ���� | ������ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
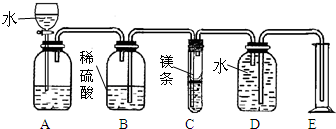
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
| 2m2g |
| m1 |
| 2m2g |
| m1 |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ��Ķ�����
 ��2013?������һģ����ǰ��������۵ļ۸����Ԫ���ϰ�Ԫ���ȣ�������ۡ��ָж�û�����Բ��죮
��2013?������һģ����ǰ��������۵ļ۸����Ԫ���ϰ�Ԫ���ȣ�������ۡ��ָж�û�����Բ��죮| ʵ������ | ���� | ���� |
| ��1���ֱ�������������ۺ���������۷����Թ��У�������ˮ������һ��ʱ��μ� ��̪��Һ ��̪��Һ �� |
��������۵��ϲ���Һ��죬��������۵���Һû�б�ɫ | ����ٳ��� |
| ��2���ֱ�������������ۺ���������۷����Թ��У�������ˮ�����ȣ� | ��������۵���Һ�л�ɫ���֣��ֲ���ڣ���������۵���Һû���������� | ����ڳ��� |
| ��������� | ��������� | |
| ����۵����� | 100 g | 100 g |
| ������������� | 460.0 g | 501.3 g |
| �ձ����������ʵ������� | 520.0 g | 557.7 g |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ��Ķ�����

| ���ϱ� | |
| ����Ƥ | ��ۡ������� |
| ���� | Ϻ�ʡ����⡢������ �ײˡ������͵� |

| ||
| ||
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ��2012�����ʡ�������п�������Ӧ�Կ��Ի�ѧ�Ծ��������棩 ���ͣ������

�鿴�𰸺ͽ���>>
����ʡ������Υ���Ͳ�����Ϣ�ٱ�ƽ̨ | �����к���Ϣ�ٱ�ר�� | ����թƭ�ٱ�ר�� | ����ʷ���������к���Ϣ�ٱ�ר�� | ������Ȩ�ٱ�ר��
Υ���Ͳ�����Ϣ�ٱ��绰��027-86699610 �ٱ����䣺58377363@163.com