
½įŗĻČēĶ¼ĖłŹ¾»Ų“šĪŹĢā£ŗ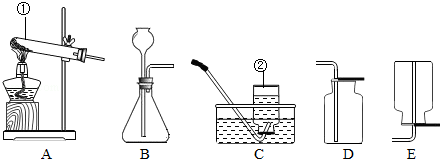
£Ø1£©Š“³öĶ¼ÖŠ±źÓŠŠņŗÅŅĒĘ÷µÄĆū³Ę¢Ł”” ”””¢¢Ś”” ””£®
£Ø2£©ÓĆ¹żŃõ»ÆĒāČÜŅŗŗĶ¶žŃõ»ÆĆĢÖĘČ”ŃõĘų£¬·“Ó¦µÄ»Æѧ·½³ĢŹ½ŹĒ”” ””£¬ĘäÖŠ¶žŃõ»ÆĆĢĘš”” ””×÷ÓĆ£¬ČōÓĆC×°ÖĆŹÕ¼ÆŃõĘų£¬¹Ū²ģµ½Ęų¹ÜæŚĘųÅŻ”” ””£¬ŌŁæŖŹ¼ŹÕ¼Æ£®
£Ø3£©Š“³öÓĆB×°ÖĆŗĶŹŹµ±Ņ©Ę·ÖĘČ”ĘųĢå£ØO2³żĶā£©µÄŅ»øö·“Ó¦»Æѧ·½³ĢŹ½”” ””£®
£Ø4£©°±Ęų¼«Ņ×ČÜÓŚĖ®£¬ĒŅĆܶȱČæÕĘųŠ”£®ŹµŃéŹŅÓĆ¼ÓČČĀČ»Æļ§ŗĶŹģŹÆ»Ņ¹ĢĢå»ģŗĻĪļµÄ·½·ØÖĘČ”°±Ęų£¬Ó¦Ń”ÓƵÄ×°ÖĆŹĒ”” ””£ØĢī×ÖÄø£©£®
£Ø1£©ŹŌ¹Ü£»¼ÆĘųĘ棻
£Ø2£©2H2O2 2H2O+O2”ü£»“߻ƣ»Į¬Šų”¢¾łŌČĆ°³ö£»
2H2O+O2”ü£»“߻ƣ»Į¬Šų”¢¾łŌČĆ°³ö£»
£Ø3£©CaCO3+2HCl=CaCl2+H2O+CO2”ü£Ø»ņZn+H2SO4=ZnSO4+H2”ü£©£»
£Ø4£©AE£®
½āĪöŹŌĢā·ÖĪö£ŗ£Ø1£©Ķ¼ÖŠ±źÓŠŠņŗÅŅĒĘ÷·Ö±šŹĒŹŌ¹ÜŗĶ¼ÆĘųĘ棻
£Ø2£©ÓĆ¹żŃõ»ÆĒāČÜŅŗŗĶ¶žŃõ»ÆĆĢÖĘČ”ŃõĘų£¬Ķ¬Ź±Éś³ÉĖ®£¬·“Ó¦µÄ»Æѧ·½³ĢŹ½ŹĒ2H2O2 2H2O+O2”ü£»øĆ·“Ó¦ÖŠ¶žŃõ»ÆĆĢæɼÓæģ·“Ó¦ĖŁĀŹ£¬Ęš“ß»Æ×÷ÓĆ£¬ÅÅĖ®·ØŹÕ¼ÆĘųĢåŅŖ“żĘųÅŻĮ¬Šų”¢¾łŌČĆ°³öŌŁæŖŹ¼ŹÕ¼Æ£¬·ĄÖ¹ĘųĢå²»“棻
2H2O+O2”ü£»øĆ·“Ó¦ÖŠ¶žŃõ»ÆĆĢæɼÓæģ·“Ó¦ĖŁĀŹ£¬Ęš“ß»Æ×÷ÓĆ£¬ÅÅĖ®·ØŹÕ¼ÆĘųĢåŅŖ“żĘųÅŻĮ¬Šų”¢¾łŌČĆ°³öŌŁæŖŹ¼ŹÕ¼Æ£¬·ĄÖ¹ĘųĢå²»“棻
£Ø3£©B×°ÖĆŹŹÓĆÓŚ¹ĢĢåŗĶŅŗĢå³£ĪĀ·“Ó¦ÖĘČ”ĘųĢ壬ŹµŃéŹŅĄūÓĆ“óĄķŹÆŗĶĻ”ŃĪĖį·“Ó¦ÖĘČ”¶žŃõ»ÆĢ¼£¬ĄūÓĆŠæŗĶĻ”ĮņĖį·“Ó¦ÖĘČ”ĒāĘų¾ł²»Šč¼ÓČČ£¬æÉÓĆ“Ė·¢Éś×°ÖĆ£¬·½³ĢŹ½·Ö±šŹĒ£ŗCaCO3+2HCl=CaCl2+H2O+CO2”ü”¢Zn+H2SO4=ZnSO4+H2”ü£»
£Ø4£©ŹµŃéŹŅÓĆ¼ÓČČĀČ»Æļ§ŗĶŹģŹÆ»Ņ¹ĢĢå»ģŗĻĪļµÄ·½·ØÖĘČ”°±Ęų£¬ŹōÓŚ¹ĢĢå¼ÓČČŠĶ£¬¹ŹŃ”·¢Éś×°ÖĆA£¬°±Ęų¼«Ņ×ČÜÓŚĖ®£¬ĒŅĆܶȱČæÕĘųŠ”£¬¹ŹÖ»ÄÜÓĆĻņĻĀÅÅæÕĘų·ØŹÕ¼Æ£»
æ¼µć£ŗ³£ÓĆĘųĢåµÄ·¢Éś×°ÖĆŗĶŹÕ¼Æ×°ÖĆÓėєȔ·½·Ø£»ŹµŃéŹŅÖĘČ”ŃõĘųµÄ·“Ó¦ŌĄķ£»ÖĘČ”ŃõĘųµÄ²Ł×÷²½ÖčŗĶ×¢Ņāµć£»ŹéŠ“»Æѧ·½³ĢŹ½”¢ĪÄ×Ö±ķ“ļŹ½”¢µēĄė·½³ĢŹ½£®
µćĘĄ£ŗÕĘĪÕŹµŃéŹŅÖĘČ”ŃõĘų”¢¶žŃõ»ÆĢ¼”¢ĒāĘųµÄ·½·Ø£¬·¢ÉśŗĶŹÕ¼Æ×°ÖƵÄŃ”Ōń±ź×¼£¬¼°ŹµŃé×¢ŅāŹĀĻīµČÖŖŹ¶£¬²ÅÄÜŅĄ¾ŻĢāŅāÕżČ··ÖĪö½ā“š£®



| Äź¼¶ | øßÖŠæĪ³Ģ | Äź¼¶ | ³õÖŠæĪ³Ģ |
| øßŅ» | øßŅ»Ćā·ŃæĪ³ĢĶĘ¼ö£” | ³õŅ» | ³õŅ»Ćā·ŃæĪ³ĢĶĘ¼ö£” |
| ø߶ž | ø߶žĆā·ŃæĪ³ĢĶĘ¼ö£” | ³õ¶ž | ³õ¶žĆā·ŃæĪ³ĢĶĘ¼ö£” |
| øßČż | øßČżĆā·ŃæĪ³ĢĶĘ¼ö£” | ³õČż | ³õČżĆā·ŃæĪ³ĢĶĘ¼ö£” |
æĘÄæ£ŗ³õÖŠ»Æѧ Ą“Ō“£ŗ ĢāŠĶ£ŗĢīæÕĢā
Ķ¼ŹĒŹµŃéŹŅ³£ÓƵÄÖĘČ”ĘųĢåµÄ×°ÖĆ£¬»Ų“šÓŠ¹ŲĪŹĢā£ŗ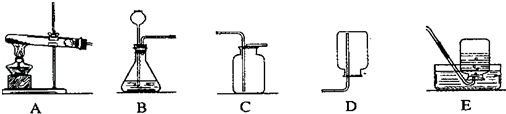
£Ø1£©A×°ÖĆÖŠŹŌ¹ÜæŚ±ŲŠėĻņĻĀĒ抱µÄŌŅņŹĒ ”£
£Ø2£©ÓĆEŹÕ¼ÆµÄĘųĢå±ŲŠė¾ß±øµÄŠŌÖŹŹĒ ”£
£Ø3£©ÓĆøßĆĢĖį¼ŲÖĘČ”ŃõĘųӦєŌńµÄ·¢Éś×°ÖĆŹĒ£ØĢī×°ÖĆŠņŗÅ£© ”£
£Ø4£©Š“³öŹµŃéŹŅæÉÓĆB”¢C×°ÖĆ×éŗĻĄ“ÖĘČ”µÄŅ»ÖÖĘųĢåµÄѧ·½³ĢŹ½ĪŖ ”£
²éæ““š°øŗĶ½āĪö>>
æĘÄæ£ŗ³õÖŠ»Æѧ Ą“Ō“£ŗ ĢāŠĶ£ŗĪŹ“šĢā
øł¾ŻČēĶ¼ĖłŹ¾×°ÖĆ£¬½įŗĻĖłŃ§ÖŖŹ¶»Ų“šĻĀĮŠĪŹĢā£®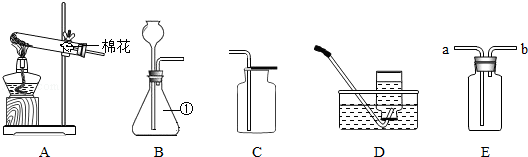
£Ø1£©Š“³öĶ¼ÖŠŅĒĘ÷¢ŁµÄĆū³Ę £»
£Ø2£©ŹµŃéŹŅÓĆA×°ÖĆÖĘČ”ŃõĘų£¬Š“³ö·“Ó¦µÄ»Æѧ·½³ĢŹ½ £»
£Ø3£©ŹµŃéŹŅÓĆB”¢E”¢C×°ÖĆÖĘČ”²¢ŹÕ¼ÆøÉŌļµÄŃõĘų£¬ŌņEÖŠÓ¦Ź¢·ÅµÄŹŌ¼ĮŹĒ £»
£Ø4£©ŹµŃéŹŅÓĆD×°ÖĆŹÕ¼ÆŃõĘų£¬µ± Ź±æŖŹ¼ŹÕ¼Æ£»
£Ø5£©E×°ÖĆÖŠ³äĀśĖ®Ź±£¬Ņ²æÉŅŌ×÷ĪŖŃõĘųµÄŹÕ¼Æ×°ÖĆ£¬ŌņŃõĘųÓ¦“Ó ¶ĖĶØČė£ØĢī”°a”±»ņ”°b”±£©£®
²éæ““š°øŗĶ½āĪö>>
æĘÄæ£ŗ³õÖŠ»Æѧ Ą“Ō“£ŗ ĢāŠĶ£ŗĪŹ“šĢā
ČēĶ¼ĖłŹ¾ŹĒ³õÖŠ»ÆѧŹµŃéŹŅÖŠ³£¼ūµÄ¼øÖÖŅĒĘ÷£¬Ēė°“ŅŖĒó»Ų“šĻĀĮŠĪŹĢā£®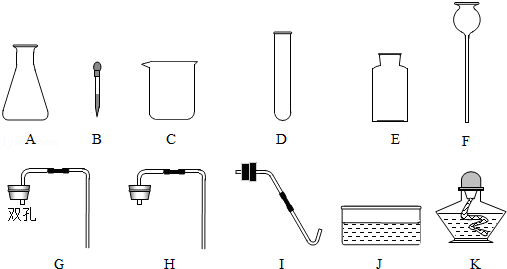
£Ø1£©Ķ¼ÖŠŅĒĘ÷CµÄĆū³ĘŹĒ”” ””£»ŅĒĘ÷FµÄĆū³ĘŹĒ”” ””£®
£Ø2£©ŌŚŹµŃéŹŅÖĘČ”¶žŃõ»ÆĢ¼£¬×īŗĆŃ”ŌńĻĀĮŠŅ©Ę·ÖŠµÄ”” ””£ØĢīŃ”Ļī×ÖÄø£©£®
a£®Ģ¼ĖįøĘ£»b£®“óĄķŹÆ£»c£®Ļ”ŃĪĖį£»d£®Ļ”ĮņĖį£®
£Ø3£©Ń”ŌńA”¢FŗĶ”” ””£ØĢī×ÖÄø£©æÉ×é×°³ÉŅ»Ģ׏µŃéŹŅÖĘČ”ŃõĘųµÄ·¢Éś×°ÖĆŗĶŹÕ¼Æ×°ÖĆ£®Š“³öÓĆøĆ×°ÖĆÖĘČ”ŃõĘųµÄ»Æѧ·½³ĢŹ½”” ””£®
²éæ““š°øŗĶ½āĪö>>
æĘÄæ£ŗ³õÖŠ»Æѧ Ą“Ō“£ŗ ĢāŠĶ£ŗĪŹ“šĢā
ŹµŃéŹŅÖĘČ”Ä³Š©ĘųĢåµÄ×°ÖĆĶ¼ČēĶ¼ĖłŹ¾£®Ēė°“ŅŖĒó»Ų“šĻĀĮŠĪŹĢā£®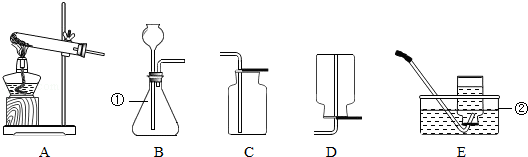
£Ø1£©Š“³öÉĻĶ¼ĖłŹ¾×°ÖĆÖŠ±źÓŠŠņŗŵÄŅĒĘ÷Ćū³Ę£ŗ¢Ł”” ””£»¢Ś”” ””£®
£Ø2£©ŹµŃéŹŅÓĆøßĆĢĖį¼ŲÖĘČ”ŃõĘųµÄ»Æѧ·½³ĢŹ½ĪŖ”” ””£»ÖĘČ”ŃõĘųŹ±ŹŌ¹ÜæŚĪ“·ÅĆŽ»Ø£¬Ęäŗó¹ūŹĒ”” ””£®
£Ø3£©ŹµŃéŹŅÖĘČ”ŗĶŹÕ¼Æ¶žŃõ»ÆĢ¼Ó¦Ń”ŌńµÄ×°ÖĆĪŖ”” ””£ØĢī×ÖÄø£©£¬Ń”ŌńøĆŹÕ¼Æ×°ÖƵĥķÓÉŹĒ”” ””£®
²éæ““š°øŗĶ½āĪö>>
æĘÄæ£ŗ³õÖŠ»Æѧ Ą“Ō“£ŗ ĢāŠĶ£ŗĪŹ“šĢā
ÓĆĶ¼1ŗĶĶ¼2ĖłŹ¾×°ÖĆ·Ö±šÖĘČ”ŹŹĮæO2ŗĶCO2ĘųĢ壮Ēė»Ų“šĻĀĮŠĪŹĢā£®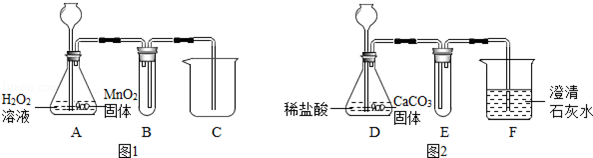
£Ø1£©AÖŠMnO2µÄ×÷ÓĆŹĒ”” ””£®
£Ø2£©Š“³öA”¢DÖŠ·¢Éś·“Ó¦µÄ»Æѧ·½³ĢŹ½£¬A£ŗ”” ””£»D£ŗ”” ””£®
£Ø3£©°“ŹµŃéŅŖĒóĶź³ÉĻĀ±ķÄŚČŻ£Ø²»ŠčŅŖµÄæÉŅŌ²»Ģī£©£®
²éæ““š°øŗĶ½āĪö>>
æĘÄæ£ŗ³õÖŠ»Æѧ Ą“Ō“£ŗ ĢāŠĶ£ŗĢ½¾æĢā
ĻĀĮŠĮ½×é×°ÖĆĶ¼¶¼ÓėĪŅĆĒѧ¹żµÄĘųĢåµÄÖĘČ”ŗĶŠŌÖŹÓŠ¹Ų”£
£Ø1£©øł¾ŻĻĀĶ¼£ØA”«E£©ĖłøųµÄ×°ÖĆ£¬»Ų“šĻĀĮŠĪŹĢā£ŗ
ŹµŃéŹŅÓĆøßĆĢĖį¼ŲÖĘČ”ŗĶŹÕ¼ÆŃõĘų£¬æÉŃ”ÓƵÄ×°ÖĆĪŖ £ØĢī×ÖÄø£©£¬·“Ó¦·½³ĢŹ½ĪŖ £»ŹµŃéŹŅÓĆ¹żŃõ»ÆĒāÖĘČ”ŗĶŹÕ¼ÆŃõĘų£¬æÉŃ”ÓƵÄ×°ÖĆĪŖ £ØĢī×ÖÄø£©£¬·“Ó¦·½³ĢŹ½ĪŖ £»ŹµŃéŹŅÖĘČ”ŗĶŹÕ¼Æ¶žŃõ»ÆĢ¼£¬æÉŃ”ÓƵÄ×°ÖĆĪŖ £ØĢī×ÖÄø£©£¬·“Ó¦·½³ĢŹ½ĪŖ ”£
£Ø2£©ŌŚŹµŃéŹŅÖŠ£¬¼ÆĘųĘæ±»³Ę×ö”°ĶņÄÜĘæ”±£¬ÓĆĖüæÉŅŌ×é×°³Éø÷ÖÖÓĆĶ¾µÄ×°ÖĆ£¬Ēė׊Ļø¹Ū²ģĻĀĮŠ£ØF”«K£©ø÷øö×°ÖƵÄĢŲµć»Ų“šĻĀĮŠĪŹĢā£ŗ 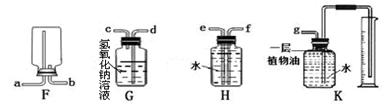
¢ŁŹµŃéŹŅČōÓĆF×°ÖĆŹÕ¼ÆŃõĘų£¬ŌņŃõĘųÓ¦“Ó æŚ½ųČė£ØĢīµ¼¹ÜæŚµÄ×ÖÄø“śŗÅ£©”£
¢ŚČōŅŖŹÕ¼ÆŅ»¶ØĢå»żµÄ¶žŃõ»ÆĢ¼ĘųĢå£¬Ó¦Ń”ÓĆ ×°ÖĆ”£
¢ŪČōŅŖ³żČ„Ņ»Ńõ»ÆĢ¼ÖŠµÄÉŁĮ涞Ńõ»ÆĢ¼£¬Ķ¬Ź±ÓĆøĆĘæŹÕ¼Æ½Ļ“æ¾»µÄŅ»Ńõ»ÆĢ¼£¬Ó¦Ń”ÓĆ ×°ÖĆ”£
²éæ““š°øŗĶ½āĪö>>
æĘÄæ£ŗ³õÖŠ»Æѧ Ą“Ō“£ŗ ĢāŠĶ£ŗĢ½¾æĢā
£Ø6·Ö£©ČēĶ¼ĖłŹ¾ĪŖŹµŃéŹŅÖŠ³£¼ūµÄĘųĢåÖʱø”¢¾»»Æ”¢ŹÕ¼ÆŗĶŠŌÖŹŹµŃéµÄ²æ·ÖŅĒĘ÷”£ŹŌøł¾ŻĢāÄæŅŖĒ󣬻Ų“šĻĀĮŠĪŹĢā£ŗ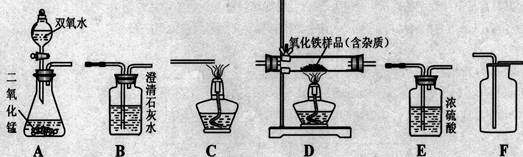
£Ø1£© ÓūŌŚŹµŃéŹŅÖŠÖʱø²¢ŹÕ¼ÆøÉŌļµÄŃõĘų”£
¢Ł ĖłŃ”ŅĒĘ÷µÄĮ¬½ÓĖ³ŠņĪŖ (ĢīŠ“ŅĒĘ÷ŠņŗÅ×ÖÄø)”£
¢ŚŅĒĘ÷AÖŠ£¬·¢Éś·“Ó¦µÄ»Æѧ·½³ĢŹ½ĪŖ ”£
£Ø2£© ÓūÓĆ×ćĮæ“æ¾»µÄŅ»Ńõ»ÆĢ¼ĘųĢå²ā¶Øij²»“æŃõ»ÆĢśŃłĘ·µÄ“æ¶Č (ŌÓÖŹ²»·“Ó¦)£¬²¢ŃéÖ¤·“Ó¦ÖŠĘųĢåÉś³ÉĪļµÄŠŌÖŹ”£ĖłŃ”ŅĒĘ÷µÄĮ¬½ÓĖ³ŠņĪŖ£ŗ“æ¾»µÄŅ»Ńõ»ÆĢ¼ĘųĢå ”śD”śB”śC”£
¢ŁŅĒĘ÷CµÄ×÷ÓĆŹĒ ”£
¢ŚŅĒĘ÷DÖŠ·¢ÉśµÄ»Æѧ·“Ó¦·½³ĢŹ½ĪŖ
¢Ū·“Ó¦ĶźČ«ŗ󣬼ĘĖćŃõ»ÆĢśŃłĘ·µÄ“æ¶ČŹ±£¬Š”ĒæĶ¬Ń§ČĻĪŖ”°ŅĒĘ÷DÖŠ¼õÉŁµÄÖŹĮæµČÓŚŅĒĘ÷BÖŠŌö¼ÓµÄÖŹĮæ”±”£Š”ĄöĶ¬Ń§Ķعż³ĘĮæ·¢ĻÖ¶žÕßÖŹĮæ²¢²»ĻąµČ”£ĒėÄć·ÖĪöŠ“³öŅĒĘ÷DÖŠ¼õÉŁµÄŹĒ ________µÄÖŹĮ棬¶ųŅĒĘ÷BÖŠŌö¼ÓµÄŹĒ _________µÄÖŹĮ攣
²éæ““š°øŗĶ½āĪö>>
æĘÄæ£ŗ³õÖŠ»Æѧ Ą“Ō“£ŗ ĢāŠĶ£ŗĢ½¾æĢā
ŹµŃéŹŅÖĘČ”Ä³Š©ĘųĢåĖłŠčµÄ×°ÖĆČēĶ¼ĖłŹ¾£¬Ēė»Ų“šĻĀĮŠĪŹĢā£®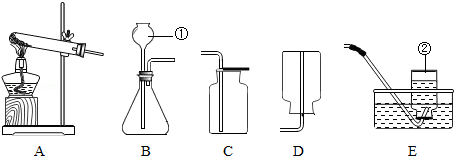
£Ø1£©ŅĒĘ÷Ćū³Ę£ŗ¢Ł”” ””£¬¢Ś”” ””£®
£Ø2£©Ń”ÓĆA×°ÖĆÖĘČ”ŃõĘųµÄŅ»øö»Æѧ·½³ĢŹ½ĪŖ”” ””£¬ŹÕ¼ÆŃõĘųæÉŃ”ÓƵÄŅ»ÖÖ×°ÖĆŹĒ”” ””£ØĢī×ÖÄø£©£®
£Ø3£©ŹµŃéŹŅÖĘČ”¶žŃõ»ÆĢ¼ĘųĢåӦєŌńµÄ·¢Éś×°ÖĆŹĒ”” ””£ØĢī×ÖÄø£©£¬ÖĘČ”¶žŃõ»ÆĢ¼µÄ»Æѧ·½³ĢŹ½ĪŖ”” ””£®
²éæ““š°øŗĶ½āĪö>>
°Ł¶ČÖĀŠÅ - Į·Ļ°²įĮŠ±ķ - ŹŌĢāĮŠ±ķ
ŗž±±Ź”»„ĮŖĶųĪ„·ØŗĶ²»Į¼ŠÅĻ¢¾Ł±ØĘ½ĢØ | ĶųÉĻÓŠŗ¦ŠÅĻ¢¾Ł±Ø×ØĒų | µēŠÅÕ©Ę¾Ł±Ø×ØĒų | É꥜Ź·ŠéĪŽÖ÷ŅåÓŠŗ¦ŠÅĻ¢¾Ł±Ø×ØĒų | ÉęĘóĒÖČؾŁ±Ø×ØĒų
Ī„·ØŗĶ²»Į¼ŠÅĻ¢¾Ł±Øµē»°£ŗ027-86699610 ¾Ł±ØÓŹĻä£ŗ58377363@163.com