
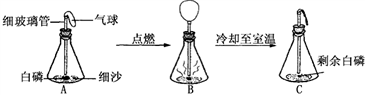
���ð���(��������������ͬ���Ż��Ϊ40��)̽�������غ㶨�ɵ�ʵ���У�����ͬѧ����Ƥ���ϵ�ϸ�������¶˷ŵ��ƾ��ƻ��������������Ⱥ�Ѹ������Ƥ������ƿ����������ȼ���ף�ʵ���������ͼ��ʾ����ش��������⣺

(1)ʵ��۲쵽A��C������Ĵ�С��ͬ��������֪ʶ���Ͳ����������ԭ����
��
(2)��C�ٴηŵ���ƽ�ϳ�������ƽ��Ȼƽ�⣬�ڴ˻�ѧ��Ӧ�У��Ӿ���ķ�Ӧ��������������ƽƽ���ԭ���� ��
77��(�����)д�����з�Ӧ�Ļ�ѧ����ʽ��
(1)���������г��ȼ��������������������������
(2)ˮ��ͨ�������·ֽ�������������������������
(3)��������ͭ��Һ��Ӧ������������������������
(4)����������Һ��ϡ���ᷴӦ����������������������
 ��������һ���þ�ϵ�д�
��������һ���þ�ϵ�д� Сѧ��10����Ӧ����ϵ�д�
Сѧ��10����Ӧ����ϵ�д�
| �꼶 | ���пγ� | �꼶 | ���пγ� |
| ��һ | ��һ��ѿγ��Ƽ��� | ��һ | ��һ��ѿγ��Ƽ��� |
| �߶� | �߶���ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
| ���� | ������ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
��5�֣����ð���(��������������ͬ���Ż��Ϊ40��)̽�������غ㶨�ɵ�ʵ���У�����ͬѧ����Ƥ���ϵ�ϸ�������¶˷ŵ��ƾ��ƻ��������������Ⱥ�Ѹ������Ƥ������ƿ����������ȼ���ף�ʵ���������ͼ��ʾ����ش��������⣺
(1)ʵ��۲쵽A��C������Ĵ�С��ͬ��ΪʲôC�е������ø����ˣ�ԭ����
��
(2)���A��ҩƷ���ɶ������̺�˫��ˮ���ȰѶ������̷��������У�����ƿ�������ŵ���ƽ�ϳ���������̫ƽƽ�⣬Ȼ��������еĶ������̵��뵽˫��ˮ�У���ַ�Ӧ���ٴηŵ���ƽ�ϳ�������ƽ�Ƿ���Ȼƽ�⣿ ����ƽƫ�� ��(����һ����ƿһ��)
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ��2011--2012ѧ�꽭��ʡ�γǵ���������һѧ�����п��Ի�ѧ�Ծ� ���ͣ������
��5�֣����ð���(��������������ͬ���Ż��Ϊ40��)̽�������غ㶨�ɵ�ʵ���У�����ͬѧ����Ƥ���ϵ�ϸ�������¶˷ŵ��ƾ��ƻ��������������Ⱥ�Ѹ������Ƥ������ƿ����������ȼ���ף�ʵ���������ͼ��ʾ����ش��������⣺

(1)ʵ��۲쵽A��C������Ĵ�С��ͬ��ΪʲôC�е������ø����ˣ�ԭ����
��
(2)���A��ҩƷ���ɶ������̺�˫��ˮ���ȰѶ������̷��������У�����ƿ�������ŵ���ƽ�ϳ���������̫ƽƽ�⣬Ȼ��������еĶ������̵��뵽˫��ˮ�У���ַ�Ӧ���ٴηŵ���ƽ�ϳ�������ƽ�Ƿ���Ȼƽ�⣿ ����ƽƫ�� ��(����һ����ƿһ��)
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ������ʡͬ���� ���ͣ�ʵ����
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
���ð���(��������������ͬ���Ż��Ϊ40��)̽�������غ㶨�ɵ�ʵ���У�����ͬѧ����Ƥ���ϵ�ϸ�������¶˷ŵ��ƾ��ƻ��������������Ⱥ�Ѹ������Ƥ������ƿ����������ȼ���ף�ʵ���������ͼ��ʾ����ش��������⣺

(1)ʵ��۲쵽A��C������Ĵ�С��ͬ��������֪ʶ���Ͳ����������ԭ����
��
(2)��C�ٴηŵ���ƽ�ϳ�������ƽ��Ȼƽ�⣬�ڴ˻�ѧ��Ӧ�У��Ӿ���ķ�Ӧ��������������ƽƽ���ԭ���� ��
77��(�����)д�����з�Ӧ�Ļ�ѧ����ʽ��
(1)���������г��ȼ��������������������������
(2)ˮ��ͨ�������·ֽ�������������������������
(3)��������ͭ��Һ��Ӧ������������������������
(4)����������Һ��ϡ���ᷴӦ����������������������
�鿴�𰸺ͽ���>>
����ʡ������Υ���Ͳ�����Ϣ�ٱ�ƽ̨ | �����к���Ϣ�ٱ�ר�� | ����թƭ�ٱ�ר�� | ����ʷ���������к���Ϣ�ٱ�ר�� | ������Ȩ�ٱ�ר��
Υ���Ͳ�����Ϣ�ٱ��绰��027-86699610 �ٱ����䣺58377363@163.com